|
An acid is defined as a chemical compound with a pH value less than 7, indicating a higher concentration of hydrogen ions (H⁺) than hydroxide ions (OH⁻). |
Card: 2 / 34 |
|
True. Strong acids, like HCl and H₂SO₄, dissociate completely or almost completely in water. |
Card: 4 / 34 |
|
When an acid reacts with a metal, it undergoes a displacement reaction, producing a salt and releasing hydrogen gas. |
Card: 8 / 34 |
|
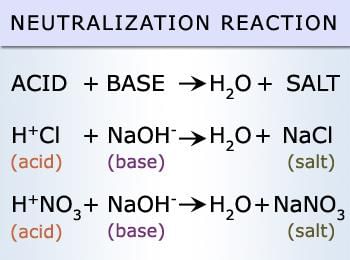
Fill in the blank: The neutralization reaction between an acid and a base produces ___ and ___. |
Card: 13 / 34 |
|
Strong bases completely ionize in water, producing a large number of hydroxide ions, while weak bases partially ionize. |
Card: 16 / 34 |
 Unlock all Flashcards with EduRev Infinity Plan Starting from @ ₹99 only
|
|
Indicators change color in the presence of acids or bases, allowing for their identification without tasting. |
Card: 20 / 34 |
|
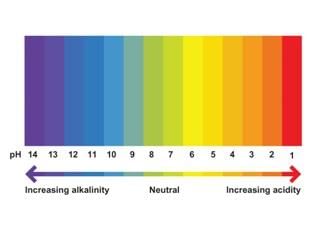
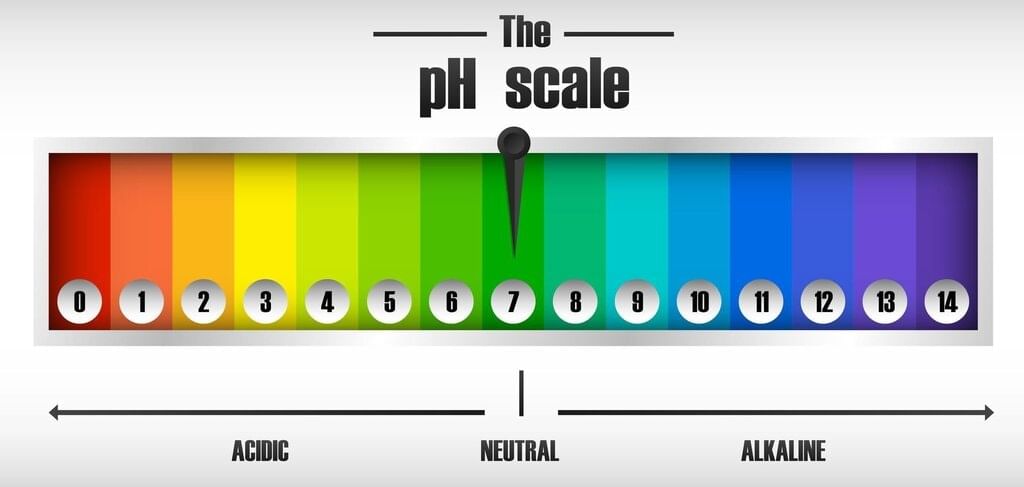
A pH less than 7 indicates an acidic solution, a pH of 7 indicates neutrality, and a pH greater than 7 indicates a basic solution. |
Card: 26 / 34 |
|
Fill in the blank: Salts formed from strong acids and strong bases have a pH of approximately ___. |
Card: 27 / 34 |
|
Bleaching powder is primarily used as a bleaching agent in the textile industry and as a disinfectant. |
Card: 34 / 34 |