Class 11 Exam > Class 11 Questions > Write the type of hybridization and structure...
Start Learning for Free
Write the type of hybridization and structure for XeOF4 and [CrF6]^3-?
Most Upvoted Answer
Write the type of hybridization and structure for XeOF4 and [CrF6]^3-?
I have used a simple never-fail approach with my students, which I call the “n+sigma” rule. Here’s what you do:
draw the Lewis structure of the compound. Here is one possible structure for XeOF4:
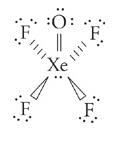
2. look at the central atom (here, Xe) and classify all the electron pairs around it as either sigma, pi, or unshared (n). Remember that single bonds are sigma, double bonds consist of one sigma and one pi bond, and triple bonds consist of one sigma and two pi bonds. Here, sigma = 5, pi = 1 and n= 1.
Add sigma + n = 5+1 = 6. this is the number of hybrid orbitals that Xe will need to house these electron pairs; so here Xe needs 6 hybrid orbitals.
3. The central atom will use its s-orbital, its p-orbitals and as many of its d-orbitals as needed to mix together to make the hybrid orbitals. So, for XeOF4, Xe will need its s orbital, all three of its p-orbitals, and 2 of its d-orbitals, and its hybridization state will be sp3d2, or d2sp3.
There is another possible structure you can draw:
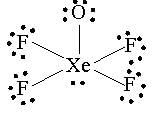
but this will still give you the same answer.
Try this approach for a number of different molecules; it works like a charm!
Community Answer
Write the type of hybridization and structure for XeOF4 and [CrF6]^3-?
Hybridization and Structure of XeOF4:
Hybridization:
The central atom in XeOF4 is xenon (Xe), which belongs to the noble gas family. Xenon has the electron configuration 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6, with eight valence electrons. In the excited state, one of the 5p electrons is promoted to the 5d orbital to achieve a total of five orbitals available for bonding. Therefore, the hybridization of xenon in XeOF4 is sp3d2.
Structure:
XeOF4 adopts a square pyramidal structure with the xenon atom at the center. The four oxygen atoms are bonded to the xenon atom in a planar arrangement, while the fluorine atom occupies the apical position, pointing away from the plane. The lone pair of electrons on the xenon atom occupies one of the equatorial positions, resulting in a distorted octahedral geometry.
Hybridization and Structure of [CrF6]^3-:
Hybridization:
The central atom in [CrF6]^3- is chromium (Cr), which belongs to the transition metal family. Chromium has the electron configuration [Ar] 3d5 4s1, with six valence electrons. In order to form six bonds, the chromium atom undergoes hybridization. The hybridization of chromium in [CrF6]^3- is sp3d2, similar to the hybridization of xenon in XeOF4.
Structure:
[CrF6]^3- adopts an octahedral structure with the chromium atom at the center. The six fluoride ions are bonded to the chromium atom, occupying the six coordination positions. The octahedral geometry is achieved due to the sp3d2 hybridization of the chromium atom, which allows for the formation of six sigma bonds.
Overall, both XeOF4 and [CrF6]^3- exhibit sp3d2 hybridization, resulting in square pyramidal and octahedral structures, respectively. These hybridization and structure arrangements are determined by the number of available orbitals for bonding and the repulsion between electron pairs, which strive to minimize energy and achieve maximum stability.
Hybridization:
The central atom in XeOF4 is xenon (Xe), which belongs to the noble gas family. Xenon has the electron configuration 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6, with eight valence electrons. In the excited state, one of the 5p electrons is promoted to the 5d orbital to achieve a total of five orbitals available for bonding. Therefore, the hybridization of xenon in XeOF4 is sp3d2.
Structure:
XeOF4 adopts a square pyramidal structure with the xenon atom at the center. The four oxygen atoms are bonded to the xenon atom in a planar arrangement, while the fluorine atom occupies the apical position, pointing away from the plane. The lone pair of electrons on the xenon atom occupies one of the equatorial positions, resulting in a distorted octahedral geometry.
Hybridization and Structure of [CrF6]^3-:
Hybridization:
The central atom in [CrF6]^3- is chromium (Cr), which belongs to the transition metal family. Chromium has the electron configuration [Ar] 3d5 4s1, with six valence electrons. In order to form six bonds, the chromium atom undergoes hybridization. The hybridization of chromium in [CrF6]^3- is sp3d2, similar to the hybridization of xenon in XeOF4.
Structure:
[CrF6]^3- adopts an octahedral structure with the chromium atom at the center. The six fluoride ions are bonded to the chromium atom, occupying the six coordination positions. The octahedral geometry is achieved due to the sp3d2 hybridization of the chromium atom, which allows for the formation of six sigma bonds.
Overall, both XeOF4 and [CrF6]^3- exhibit sp3d2 hybridization, resulting in square pyramidal and octahedral structures, respectively. These hybridization and structure arrangements are determined by the number of available orbitals for bonding and the repulsion between electron pairs, which strive to minimize energy and achieve maximum stability.

|
Explore Courses for Class 11 exam
|

|
Similar Class 11 Doubts
Write the type of hybridization and structure for XeOF4 and [CrF6]^3-?
Question Description
Write the type of hybridization and structure for XeOF4 and [CrF6]^3-? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Write the type of hybridization and structure for XeOF4 and [CrF6]^3-? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Write the type of hybridization and structure for XeOF4 and [CrF6]^3-?.
Write the type of hybridization and structure for XeOF4 and [CrF6]^3-? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Write the type of hybridization and structure for XeOF4 and [CrF6]^3-? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Write the type of hybridization and structure for XeOF4 and [CrF6]^3-?.
Solutions for Write the type of hybridization and structure for XeOF4 and [CrF6]^3-? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of Write the type of hybridization and structure for XeOF4 and [CrF6]^3-? defined & explained in the simplest way possible. Besides giving the explanation of
Write the type of hybridization and structure for XeOF4 and [CrF6]^3-?, a detailed solution for Write the type of hybridization and structure for XeOF4 and [CrF6]^3-? has been provided alongside types of Write the type of hybridization and structure for XeOF4 and [CrF6]^3-? theory, EduRev gives you an
ample number of questions to practice Write the type of hybridization and structure for XeOF4 and [CrF6]^3-? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


























