Class 11 Exam > Class 11 Questions > Briefly describe the formal charge?
Start Learning for Free
Briefly describe the formal charge?
Verified Answer
Briefly describe the formal charge?
Formal charge of FC is the difference between the number of valence electrons of each atom and the number of electrons the atom is associated with. Formal charge assumes any shared electrons are equally shared between the two bonded atoms.
Formal charge is calculated using the equation:
FC = eV - eN - eB/2
where
eV = number of valence electrons of the atom as if it were isolated from the molecule
eN = number of unbound valence electrons on the atom in the molecule
eB = number of electrons shared by the bonds to other atoms in the molecule
Formal Charge Example Calculation
For example, carbon dioxide or CO2 is a neutral molecule that has 16 valence electrons. There are three different ways to draw the Lewis structure for the molecule to determine formal charge:
The carbon atom may be joined to both oxygen atom via double bonds (carbon = 0, oxygen = 0, formal charge = 0)
The carbon atom may have a single bond with one oxygen atom and a double bond to the other oxygen atom (carbon = +1, oxygen-double = 0, oxygen-single = -1, formal charge = 0)
The carbon atom may be joined to each oxygen atom via single bonds (carbon = +2, oxygens = -1 each, formal charge = 0)
Each possibility results in a formal charge of zero, but the first choice is the best one because it predicts no charge in the molecule. This is more stable and thus is most likely.
 This question is part of UPSC exam. View all Class 11 courses
This question is part of UPSC exam. View all Class 11 courses
Most Upvoted Answer
Briefly describe the formal charge?
This concept is used to assign formal charge on a atom in molecule not the real charge.
It is charge assigned to atom which talks about whether atom is in loss or gain or with no profit.
In case of atom our investment is electron.
Formal charge means ignore the lone pair of electrons and electrons shared by that atom from valence electron then whatever is left with you is signature of formal charge.
That is, valence electron minus total lone pairs of electrons minus electrons shared by that atom only.
If we take example of O3 (ozone) molecule.
We have this lewis dot structure.
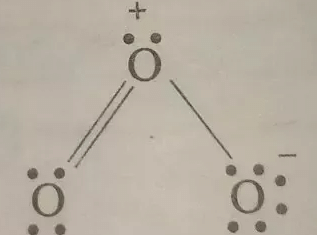
See this oxygen on which no charge is indicated.
We know that oxygen has 6 electrons in its bank account but we can see four of them are of no use and 2 are shared with another atom. What's left? Zero.
Hence formal charge on it is zero.
Now see that oxygen in figure with positive sign.
It has 6 valence electrons too, out of which 2 electron are forming one lone pair and 2 is shared with one atom and one electron minus with another atom.
So we are left with +1 .
You can make account of another left oxygen too.

|
Explore Courses for Class 11 exam
|

|
Similar Class 11 Doubts
Briefly describe the formal charge?
Question Description
Briefly describe the formal charge? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Briefly describe the formal charge? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Briefly describe the formal charge?.
Briefly describe the formal charge? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Briefly describe the formal charge? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Briefly describe the formal charge?.
Solutions for Briefly describe the formal charge? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of Briefly describe the formal charge? defined & explained in the simplest way possible. Besides giving the explanation of
Briefly describe the formal charge?, a detailed solution for Briefly describe the formal charge? has been provided alongside types of Briefly describe the formal charge? theory, EduRev gives you an
ample number of questions to practice Briefly describe the formal charge? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























