Class 11 Exam > Class 11 Questions > In an octahedral structure, the pair of d orb...
Start Learning for Free
In an octahedral structure, the pair of d orbitals involved in d2sp3 hybridization is [2004]
- a)
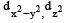
- b)
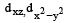
- c)dz2 , dxz
- d)dxy,dyz
Correct answer is option 'A'. Can you explain this answer?
Verified Answer
In an octahedral structure, the pair of d orbitals involved in d2sp3 h...
Only those d or bital s whose l obes are directed along X, Y and Z directions hybridise with s and p orbitals. In other three d or bitals n amely dxy, dyz and dxz, the lobes are at an angle of 45° from both axis, hence the extent of their overlap with s and p orbitals is much lesser than 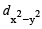 and
and 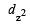 orbitals.
orbitals.
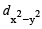 and
and 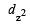 orbitals.
orbitals.Most Upvoted Answer
In an octahedral structure, the pair of d orbitals involved in d2sp3 h...
Octahedral structure and d2sp3 hybridization
Octahedral structure
An octahedral structure is a molecular geometry in which six atoms or groups of atoms are symmetrically arranged around a central atom, forming the shape of an octahedron. This structure is commonly found in coordination compounds, where a central metal ion is surrounded by six ligands.
d2sp3 hybridization
In octahedral coordination compounds, the central metal ion usually has a coordination number of six, which means it is surrounded by six ligands. In order to form six bonds, the metal ion must use its d orbitals in addition to its s and p orbitals. The hybridization of these orbitals is called d2sp3 hybridization.
Pair of d orbitals involved in d2sp3 hybridization
In d2sp3 hybridization, two of the d orbitals combine with the s and p orbitals to form four hybrid orbitals, which are arranged in an octahedral geometry. The other two d orbitals remain unhybridized and are oriented along the z-axis and the x-axis, respectively.
The pair of d orbitals involved in d2sp3 hybridization is thus:
- dx2-y2: This orbital lies along the x-axis and the y-axis and has four lobes pointing toward the corners of a square.
- dz2: This orbital lies along the z-axis and has a doughnut-shaped electron density distribution above and below the metal ion.
Therefore, the correct answer is option A, dx2-y2 and dz2.
Octahedral structure
An octahedral structure is a molecular geometry in which six atoms or groups of atoms are symmetrically arranged around a central atom, forming the shape of an octahedron. This structure is commonly found in coordination compounds, where a central metal ion is surrounded by six ligands.
d2sp3 hybridization
In octahedral coordination compounds, the central metal ion usually has a coordination number of six, which means it is surrounded by six ligands. In order to form six bonds, the metal ion must use its d orbitals in addition to its s and p orbitals. The hybridization of these orbitals is called d2sp3 hybridization.
Pair of d orbitals involved in d2sp3 hybridization
In d2sp3 hybridization, two of the d orbitals combine with the s and p orbitals to form four hybrid orbitals, which are arranged in an octahedral geometry. The other two d orbitals remain unhybridized and are oriented along the z-axis and the x-axis, respectively.
The pair of d orbitals involved in d2sp3 hybridization is thus:
- dx2-y2: This orbital lies along the x-axis and the y-axis and has four lobes pointing toward the corners of a square.
- dz2: This orbital lies along the z-axis and has a doughnut-shaped electron density distribution above and below the metal ion.
Therefore, the correct answer is option A, dx2-y2 and dz2.
Free Test
| FREE | Start Free Test |
Community Answer
In an octahedral structure, the pair of d orbitals involved in d2sp3 h...

|
Explore Courses for Class 11 exam
|

|
Question Description
In an octahedral structure, the pair of d orbitals involved in d2sp3 hybridization is [2004]a)b)c)dz2 , dxzd)dxy,dyzCorrect answer is option 'A'. Can you explain this answer? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about In an octahedral structure, the pair of d orbitals involved in d2sp3 hybridization is [2004]a)b)c)dz2 , dxzd)dxy,dyzCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In an octahedral structure, the pair of d orbitals involved in d2sp3 hybridization is [2004]a)b)c)dz2 , dxzd)dxy,dyzCorrect answer is option 'A'. Can you explain this answer?.
In an octahedral structure, the pair of d orbitals involved in d2sp3 hybridization is [2004]a)b)c)dz2 , dxzd)dxy,dyzCorrect answer is option 'A'. Can you explain this answer? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about In an octahedral structure, the pair of d orbitals involved in d2sp3 hybridization is [2004]a)b)c)dz2 , dxzd)dxy,dyzCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In an octahedral structure, the pair of d orbitals involved in d2sp3 hybridization is [2004]a)b)c)dz2 , dxzd)dxy,dyzCorrect answer is option 'A'. Can you explain this answer?.
Solutions for In an octahedral structure, the pair of d orbitals involved in d2sp3 hybridization is [2004]a)b)c)dz2 , dxzd)dxy,dyzCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of In an octahedral structure, the pair of d orbitals involved in d2sp3 hybridization is [2004]a)b)c)dz2 , dxzd)dxy,dyzCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
In an octahedral structure, the pair of d orbitals involved in d2sp3 hybridization is [2004]a)b)c)dz2 , dxzd)dxy,dyzCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for In an octahedral structure, the pair of d orbitals involved in d2sp3 hybridization is [2004]a)b)c)dz2 , dxzd)dxy,dyzCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of In an octahedral structure, the pair of d orbitals involved in d2sp3 hybridization is [2004]a)b)c)dz2 , dxzd)dxy,dyzCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice In an octahedral structure, the pair of d orbitals involved in d2sp3 hybridization is [2004]a)b)c)dz2 , dxzd)dxy,dyzCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























