Class 9 Exam > Class 9 Questions > A student heats a beaker containing ice and w...
Start Learning for Free
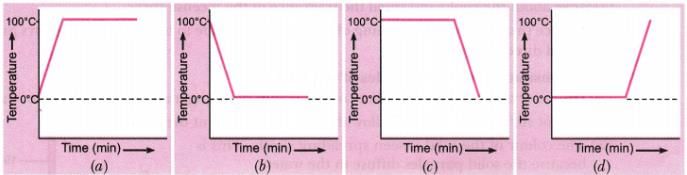
A student heats a beaker containing ice and water .He measures the temperature of the content of the beaker as a function of time. Which of the following would correctly represent the result? justify answer.
? Related: States of Matter and Its Properties
Verified Answer
A student heats a beaker containing ice and water .He measures the tem...
Since ice and water are in equilibrium, the temperature would be zero. When we heat the mixture, energy supplied is utilized in melting the ice and the temperature does not change till all the ice melts because of latent heat of fusion. On further heating, the temperature of the water would increase. Therefore, the correct option is (d)

 This question is part of UPSC exam. View all Class 9 courses
This question is part of UPSC exam. View all Class 9 courses
Most Upvoted Answer
A student heats a beaker containing ice and water .He measures the tem...
Introduction
When a student heats a beaker containing ice and water, the temperature of the content of the beaker changes over time. This change in temperature can be measured and recorded. In this response, we will explore how the temperature of the content of the beaker would change as a function of time and how it can be represented correctly.
States of Matter and Its Properties
To understand the temperature changes in the beaker, it is important to have a basic understanding of the states of matter and their properties. Matter exists in three main states: solid, liquid, and gas.
- Solid: In the solid state, particles are closely packed together and have a fixed shape and volume. The particles vibrate in place.
- Liquid: In the liquid state, particles are still close together, but they can move past each other. Liquids have a fixed volume but take the shape of their container.
- Gas: In the gas state, particles are far apart and move freely. Gases have neither a fixed shape nor volume and can expand to fill their container.
Heating the Beaker
When the student heats the beaker containing ice and water, the heat energy is transferred to the system. The ice begins to melt and the water temperature starts to increase. The temperature change can be measured over time.
Temperature of the Content of the Beaker as a Function of Time
The correct representation of the temperature of the content of the beaker as a function of time would be a graph that shows the following:
1. Initial Temperature: The temperature of the content of the beaker starts at the freezing point of water, which is 0 degrees Celsius.
2. Melting of Ice: As heat is applied, the ice in the beaker starts to melt. The temperature remains constant at 0 degrees Celsius until all the ice has melted. This is because the heat energy is used to break the intermolecular bonds holding the ice particles together rather than increasing the temperature.
3. Heating the Water: Once all the ice has melted, the temperature of the water starts to increase. The rate of temperature increase depends on the amount of heat being supplied and the specific heat capacity of water.
4. Plateau: As the water continues to heat up, it reaches a plateau at 100 degrees Celsius. This is the boiling point of water. During this phase, the heat energy is being used to convert the liquid water into water vapor instead of increasing the temperature.
5. Boiling: Once the water reaches the boiling point, it starts to boil and convert into water vapor. The temperature remains constant at 100 degrees Celsius until all the water has boiled away.
6. Vaporization: After all the water has boiled away, the temperature starts to rise again as the heat energy is now being used to increase the temperature of the water vapor.
Conclusion
In conclusion, the correct representation of the temperature of the content of the beaker as a function of time would be a graph showing the initial temperature at 0 degrees Celsius, followed by a plateau at 0 degrees Celsius during the melting of ice, an increasing temperature during the heating of water, a plateau
When a student heats a beaker containing ice and water, the temperature of the content of the beaker changes over time. This change in temperature can be measured and recorded. In this response, we will explore how the temperature of the content of the beaker would change as a function of time and how it can be represented correctly.
States of Matter and Its Properties
To understand the temperature changes in the beaker, it is important to have a basic understanding of the states of matter and their properties. Matter exists in three main states: solid, liquid, and gas.
- Solid: In the solid state, particles are closely packed together and have a fixed shape and volume. The particles vibrate in place.
- Liquid: In the liquid state, particles are still close together, but they can move past each other. Liquids have a fixed volume but take the shape of their container.
- Gas: In the gas state, particles are far apart and move freely. Gases have neither a fixed shape nor volume and can expand to fill their container.
Heating the Beaker
When the student heats the beaker containing ice and water, the heat energy is transferred to the system. The ice begins to melt and the water temperature starts to increase. The temperature change can be measured over time.
Temperature of the Content of the Beaker as a Function of Time
The correct representation of the temperature of the content of the beaker as a function of time would be a graph that shows the following:
1. Initial Temperature: The temperature of the content of the beaker starts at the freezing point of water, which is 0 degrees Celsius.
2. Melting of Ice: As heat is applied, the ice in the beaker starts to melt. The temperature remains constant at 0 degrees Celsius until all the ice has melted. This is because the heat energy is used to break the intermolecular bonds holding the ice particles together rather than increasing the temperature.
3. Heating the Water: Once all the ice has melted, the temperature of the water starts to increase. The rate of temperature increase depends on the amount of heat being supplied and the specific heat capacity of water.
4. Plateau: As the water continues to heat up, it reaches a plateau at 100 degrees Celsius. This is the boiling point of water. During this phase, the heat energy is being used to convert the liquid water into water vapor instead of increasing the temperature.
5. Boiling: Once the water reaches the boiling point, it starts to boil and convert into water vapor. The temperature remains constant at 100 degrees Celsius until all the water has boiled away.
6. Vaporization: After all the water has boiled away, the temperature starts to rise again as the heat energy is now being used to increase the temperature of the water vapor.
Conclusion
In conclusion, the correct representation of the temperature of the content of the beaker as a function of time would be a graph showing the initial temperature at 0 degrees Celsius, followed by a plateau at 0 degrees Celsius during the melting of ice, an increasing temperature during the heating of water, a plateau
Attention Class 9 Students!
To make sure you are not studying endlessly, EduRev has designed Class 9 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 9.

|
Explore Courses for Class 9 exam
|

|
Similar Class 9 Doubts
A student heats a beaker containing ice and water .He measures the temperature of the content of the beaker as a function of time. Which of the following would correctly represent the result? justify answer. Related: States of Matter and Its Properties?
Question Description
A student heats a beaker containing ice and water .He measures the temperature of the content of the beaker as a function of time. Which of the following would correctly represent the result? justify answer. Related: States of Matter and Its Properties? for Class 9 2024 is part of Class 9 preparation. The Question and answers have been prepared according to the Class 9 exam syllabus. Information about A student heats a beaker containing ice and water .He measures the temperature of the content of the beaker as a function of time. Which of the following would correctly represent the result? justify answer. Related: States of Matter and Its Properties? covers all topics & solutions for Class 9 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for A student heats a beaker containing ice and water .He measures the temperature of the content of the beaker as a function of time. Which of the following would correctly represent the result? justify answer. Related: States of Matter and Its Properties?.
A student heats a beaker containing ice and water .He measures the temperature of the content of the beaker as a function of time. Which of the following would correctly represent the result? justify answer. Related: States of Matter and Its Properties? for Class 9 2024 is part of Class 9 preparation. The Question and answers have been prepared according to the Class 9 exam syllabus. Information about A student heats a beaker containing ice and water .He measures the temperature of the content of the beaker as a function of time. Which of the following would correctly represent the result? justify answer. Related: States of Matter and Its Properties? covers all topics & solutions for Class 9 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for A student heats a beaker containing ice and water .He measures the temperature of the content of the beaker as a function of time. Which of the following would correctly represent the result? justify answer. Related: States of Matter and Its Properties?.
Solutions for A student heats a beaker containing ice and water .He measures the temperature of the content of the beaker as a function of time. Which of the following would correctly represent the result? justify answer. Related: States of Matter and Its Properties? in English & in Hindi are available as part of our courses for Class 9.
Download more important topics, notes, lectures and mock test series for Class 9 Exam by signing up for free.
Here you can find the meaning of A student heats a beaker containing ice and water .He measures the temperature of the content of the beaker as a function of time. Which of the following would correctly represent the result? justify answer. Related: States of Matter and Its Properties? defined & explained in the simplest way possible. Besides giving the explanation of
A student heats a beaker containing ice and water .He measures the temperature of the content of the beaker as a function of time. Which of the following would correctly represent the result? justify answer. Related: States of Matter and Its Properties?, a detailed solution for A student heats a beaker containing ice and water .He measures the temperature of the content of the beaker as a function of time. Which of the following would correctly represent the result? justify answer. Related: States of Matter and Its Properties? has been provided alongside types of A student heats a beaker containing ice and water .He measures the temperature of the content of the beaker as a function of time. Which of the following would correctly represent the result? justify answer. Related: States of Matter and Its Properties? theory, EduRev gives you an
ample number of questions to practice A student heats a beaker containing ice and water .He measures the temperature of the content of the beaker as a function of time. Which of the following would correctly represent the result? justify answer. Related: States of Matter and Its Properties? tests, examples and also practice Class 9 tests.

|
Explore Courses for Class 9 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























