Class 11 Exam > Class 11 Questions > Which of the following statements is true? ...
Start Learning for Free
Which of the following statements is true? [2002]
- a)Silicon exhibits 4 coordination number in its compound
- b)Bond energy of F2 is less than Cl2
- c)Mn(III) oxidation state is more stable than Mn(II) in aqueous state
- d)Elements of 15th group shows only +3 and +5 oxidation states
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Which of the following statements is true? [2002]a)Silicon exhibits...
Due to small size and high electronegativity of fluorine, there exists repulsion between the nucleus of two fluorine atoms which ultimately results in the weakening of the F-F bond. Hence, the bond dissociation enthalpy of F
2
is less than that of Cl
2
Most Upvoted Answer
Which of the following statements is true? [2002]a)Silicon exhibits...
Statement Analysis:
a) Silicon exhibits 4 coordination number in its compound
- This statement is not necessarily true as Silicon can exhibit different coordination numbers depending on the compound.
b) Bond energy of F2 is less than Cl2
- This statement is true as the bond energy of F2 is lower than that of Cl2 due to the smaller size of fluorine atoms resulting in stronger repulsion between the electrons.
c) Mn(III) oxidation state is more stable than Mn(II) in aqueous state
- This statement is not necessarily true as the stability of oxidation states depends on various factors such as pH, ligands, and temperature.
d) Elements of 15th group shows only 3 and 5 oxidation states
- This statement is not true as elements of the 15th group can exhibit oxidation states ranging from -3 to +5.
Answer Explanation:
The correct answer is option B, which states that the bond energy of F2 is less than Cl2. This is because the smaller size of fluorine atoms results in stronger repulsion between the electrons, leading to a lower bond energy compared to Cl2.
It is important to note that the other statements are either not necessarily true or completely false. Silicon can exhibit different coordination numbers in its compounds, the stability of Mn oxidation states depends on various factors, and elements of the 15th group can exhibit oxidation states ranging from -3 to +5.
a) Silicon exhibits 4 coordination number in its compound
- This statement is not necessarily true as Silicon can exhibit different coordination numbers depending on the compound.
b) Bond energy of F2 is less than Cl2
- This statement is true as the bond energy of F2 is lower than that of Cl2 due to the smaller size of fluorine atoms resulting in stronger repulsion between the electrons.
c) Mn(III) oxidation state is more stable than Mn(II) in aqueous state
- This statement is not necessarily true as the stability of oxidation states depends on various factors such as pH, ligands, and temperature.
d) Elements of 15th group shows only 3 and 5 oxidation states
- This statement is not true as elements of the 15th group can exhibit oxidation states ranging from -3 to +5.
Answer Explanation:
The correct answer is option B, which states that the bond energy of F2 is less than Cl2. This is because the smaller size of fluorine atoms results in stronger repulsion between the electrons, leading to a lower bond energy compared to Cl2.
It is important to note that the other statements are either not necessarily true or completely false. Silicon can exhibit different coordination numbers in its compounds, the stability of Mn oxidation states depends on various factors, and elements of the 15th group can exhibit oxidation states ranging from -3 to +5.
Free Test
| FREE | Start Free Test |
Community Answer
Which of the following statements is true? [2002]a)Silicon exhibits...
This is because of inter-electronic replusions between lone pairs.
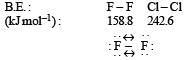
Attention Class 11 Students!
To make sure you are not studying endlessly, EduRev has designed Class 11 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 11.

|
Explore Courses for Class 11 exam
|

|
Similar Class 11 Doubts
Which of the following statements is true? [2002]a)Silicon exhibits 4 coordination number in its compoundb)Bond energy of F2 is less than Cl2c)Mn(III) oxidation state is more stable than Mn(II) in aqueous stated)Elements of 15th group shows only +3 and +5 oxidation statesCorrect answer is option 'B'. Can you explain this answer?
Question Description
Which of the following statements is true? [2002]a)Silicon exhibits 4 coordination number in its compoundb)Bond energy of F2 is less than Cl2c)Mn(III) oxidation state is more stable than Mn(II) in aqueous stated)Elements of 15th group shows only +3 and +5 oxidation statesCorrect answer is option 'B'. Can you explain this answer? for Class 11 2024 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Which of the following statements is true? [2002]a)Silicon exhibits 4 coordination number in its compoundb)Bond energy of F2 is less than Cl2c)Mn(III) oxidation state is more stable than Mn(II) in aqueous stated)Elements of 15th group shows only +3 and +5 oxidation statesCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 11 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following statements is true? [2002]a)Silicon exhibits 4 coordination number in its compoundb)Bond energy of F2 is less than Cl2c)Mn(III) oxidation state is more stable than Mn(II) in aqueous stated)Elements of 15th group shows only +3 and +5 oxidation statesCorrect answer is option 'B'. Can you explain this answer?.
Which of the following statements is true? [2002]a)Silicon exhibits 4 coordination number in its compoundb)Bond energy of F2 is less than Cl2c)Mn(III) oxidation state is more stable than Mn(II) in aqueous stated)Elements of 15th group shows only +3 and +5 oxidation statesCorrect answer is option 'B'. Can you explain this answer? for Class 11 2024 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Which of the following statements is true? [2002]a)Silicon exhibits 4 coordination number in its compoundb)Bond energy of F2 is less than Cl2c)Mn(III) oxidation state is more stable than Mn(II) in aqueous stated)Elements of 15th group shows only +3 and +5 oxidation statesCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 11 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following statements is true? [2002]a)Silicon exhibits 4 coordination number in its compoundb)Bond energy of F2 is less than Cl2c)Mn(III) oxidation state is more stable than Mn(II) in aqueous stated)Elements of 15th group shows only +3 and +5 oxidation statesCorrect answer is option 'B'. Can you explain this answer?.
Solutions for Which of the following statements is true? [2002]a)Silicon exhibits 4 coordination number in its compoundb)Bond energy of F2 is less than Cl2c)Mn(III) oxidation state is more stable than Mn(II) in aqueous stated)Elements of 15th group shows only +3 and +5 oxidation statesCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of Which of the following statements is true? [2002]a)Silicon exhibits 4 coordination number in its compoundb)Bond energy of F2 is less than Cl2c)Mn(III) oxidation state is more stable than Mn(II) in aqueous stated)Elements of 15th group shows only +3 and +5 oxidation statesCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which of the following statements is true? [2002]a)Silicon exhibits 4 coordination number in its compoundb)Bond energy of F2 is less than Cl2c)Mn(III) oxidation state is more stable than Mn(II) in aqueous stated)Elements of 15th group shows only +3 and +5 oxidation statesCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for Which of the following statements is true? [2002]a)Silicon exhibits 4 coordination number in its compoundb)Bond energy of F2 is less than Cl2c)Mn(III) oxidation state is more stable than Mn(II) in aqueous stated)Elements of 15th group shows only +3 and +5 oxidation statesCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of Which of the following statements is true? [2002]a)Silicon exhibits 4 coordination number in its compoundb)Bond energy of F2 is less than Cl2c)Mn(III) oxidation state is more stable than Mn(II) in aqueous stated)Elements of 15th group shows only +3 and +5 oxidation statesCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which of the following statements is true? [2002]a)Silicon exhibits 4 coordination number in its compoundb)Bond energy of F2 is less than Cl2c)Mn(III) oxidation state is more stable than Mn(II) in aqueous stated)Elements of 15th group shows only +3 and +5 oxidation statesCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























