Class 12 Exam > Class 12 Questions > N unknown compound on ozonolysis gives acetal...
Start Learning for Free
N unknown compound on ozonolysis gives acetaldehyde and benzophenone. The compound is?
Most Upvoted Answer
N unknown compound on ozonolysis gives acetaldehyde and benzophenone. ...
The compound that undergoes ozonolysis to produce acetaldehyde and benzophenone is called benzaldehyde.
Explanation:
1. Ozonolysis: Ozonolysis is a chemical reaction that involves the cleavage of carbon-carbon double bonds or triple bonds using ozone (O3) as the oxidizing agent. This reaction is commonly used to identify the structure of unknown compounds.
2. Products: In this case, the ozonolysis of the unknown compound results in the formation of two products: acetaldehyde and benzophenone.
3. Acetaldehyde: Acetaldehyde is a simple aldehyde with the chemical formula CH3CHO. It is a colorless liquid with a pungent odor. Acetaldehyde is formed when the carbon-carbon double bond in the unknown compound is cleaved by ozone. One of the resulting fragments reacts with ozone to form acetaldehyde.
4. Benzophenone: Benzophenone is a ketone with the chemical formula (C6H5)2CO. It is a white crystalline solid with a characteristic odor. Benzophenone is formed when the other fragment resulting from the cleavage of the carbon-carbon double bond reacts with ozone. The reaction leads to the formation of a carbonyl group, resulting in the formation of benzophenone.
5. Benzaldehyde: Benzaldehyde is an aromatic aldehyde with the chemical formula C6H5CHO. It is a colorless liquid with a characteristic almond-like odor. Benzaldehyde is the compound that undergoes ozonolysis to produce acetaldehyde and benzophenone. The carbon-carbon double bond in benzaldehyde is cleaved by ozone, resulting in the formation of these two products.
Conclusion:
In summary, the unknown compound that gives acetaldehyde and benzophenone upon ozonolysis is benzaldehyde. The carbon-carbon double bond in benzaldehyde is cleaved by ozone, leading to the formation of acetaldehyde and benzophenone as the major products.
Explanation:
1. Ozonolysis: Ozonolysis is a chemical reaction that involves the cleavage of carbon-carbon double bonds or triple bonds using ozone (O3) as the oxidizing agent. This reaction is commonly used to identify the structure of unknown compounds.
2. Products: In this case, the ozonolysis of the unknown compound results in the formation of two products: acetaldehyde and benzophenone.
3. Acetaldehyde: Acetaldehyde is a simple aldehyde with the chemical formula CH3CHO. It is a colorless liquid with a pungent odor. Acetaldehyde is formed when the carbon-carbon double bond in the unknown compound is cleaved by ozone. One of the resulting fragments reacts with ozone to form acetaldehyde.
4. Benzophenone: Benzophenone is a ketone with the chemical formula (C6H5)2CO. It is a white crystalline solid with a characteristic odor. Benzophenone is formed when the other fragment resulting from the cleavage of the carbon-carbon double bond reacts with ozone. The reaction leads to the formation of a carbonyl group, resulting in the formation of benzophenone.
5. Benzaldehyde: Benzaldehyde is an aromatic aldehyde with the chemical formula C6H5CHO. It is a colorless liquid with a characteristic almond-like odor. Benzaldehyde is the compound that undergoes ozonolysis to produce acetaldehyde and benzophenone. The carbon-carbon double bond in benzaldehyde is cleaved by ozone, resulting in the formation of these two products.
Conclusion:
In summary, the unknown compound that gives acetaldehyde and benzophenone upon ozonolysis is benzaldehyde. The carbon-carbon double bond in benzaldehyde is cleaved by ozone, leading to the formation of acetaldehyde and benzophenone as the major products.
Community Answer
N unknown compound on ozonolysis gives acetaldehyde and benzophenone. ...
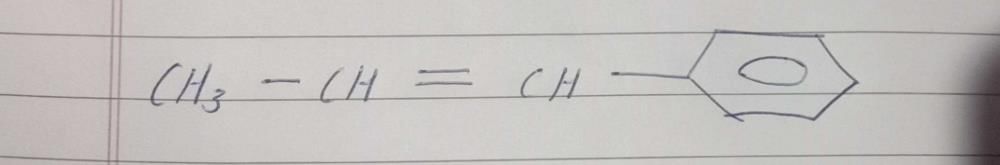

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
N unknown compound on ozonolysis gives acetaldehyde and benzophenone. The compound is?
Question Description
N unknown compound on ozonolysis gives acetaldehyde and benzophenone. The compound is? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about N unknown compound on ozonolysis gives acetaldehyde and benzophenone. The compound is? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for N unknown compound on ozonolysis gives acetaldehyde and benzophenone. The compound is?.
N unknown compound on ozonolysis gives acetaldehyde and benzophenone. The compound is? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about N unknown compound on ozonolysis gives acetaldehyde and benzophenone. The compound is? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for N unknown compound on ozonolysis gives acetaldehyde and benzophenone. The compound is?.
Solutions for N unknown compound on ozonolysis gives acetaldehyde and benzophenone. The compound is? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of N unknown compound on ozonolysis gives acetaldehyde and benzophenone. The compound is? defined & explained in the simplest way possible. Besides giving the explanation of
N unknown compound on ozonolysis gives acetaldehyde and benzophenone. The compound is?, a detailed solution for N unknown compound on ozonolysis gives acetaldehyde and benzophenone. The compound is? has been provided alongside types of N unknown compound on ozonolysis gives acetaldehyde and benzophenone. The compound is? theory, EduRev gives you an
ample number of questions to practice N unknown compound on ozonolysis gives acetaldehyde and benzophenone. The compound is? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.



















