Class 12 Exam > Class 12 Questions > Clemmensen reduction of a ketone is carried o...
Start Learning for Free
Clemmensen reduction of a ketone is carried outin the presence of which of the following ? [2011]
- a)Glycol with KOH
- b)Zn-Hg with HCl
- c)Li Al H4
- d)H2 and Pt as catalyst
Correct answer is option 'B'. Can you explain this answer?
Verified Answer
Clemmensen reduction of a ketone is carried outin the presence of whic...
Clemmensen reduction is
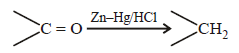
Most Upvoted Answer
Clemmensen reduction of a ketone is carried outin the presence of whic...
Clemmensen Reduction and Its Mechanism:
The Clemmensen reduction is a chemical reaction that involves the conversion of a carbonyl group in a ketone to a methylene group using zinc amalgam and hydrochloric acid. This process is particularly effective for reducing ketones to alkanes.
Role of Zn-Hg with HCl:
- In the Clemmensen reduction, zinc amalgam (Zn-Hg) serves as the reducing agent while hydrochloric acid (HCl) acts as a catalyst.
- The reaction occurs at high temperatures, typically refluxing conditions, to facilitate the reduction of the carbonyl group.
- The zinc amalgam reacts with the carbonyl group in the ketone, leading to the formation of an alkyl zinc intermediate.
- The alkyl zinc intermediate is unstable and undergoes protonation by HCl to form the corresponding alkane.
Comparison with Other Reagents:
- Glycol with KOH is commonly used for cleaving ethers, not for the Clemmensen reduction of ketones.
- LiAlH4 is a powerful reducing agent that can reduce ketones to alcohols, but it is not typically used in the Clemmensen reduction.
- H2 and Pt as a catalyst are commonly used for hydrogenation reactions, not for the Clemmensen reduction.
In conclusion, the Clemmensen reduction of a ketone is carried out in the presence of zinc amalgam and hydrochloric acid. These reagents work together to reduce the carbonyl group in the ketone to a methylene group, producing the desired alkane product.

|
Explore Courses for Class 12 exam
|

|
Question Description
Clemmensen reduction of a ketone is carried outin the presence of which of the following ?[2011]a)Glycol with KOHb)Zn-Hg with HClc)Li Al H4d)H2 and Pt as catalystCorrect answer is option 'B'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Clemmensen reduction of a ketone is carried outin the presence of which of the following ?[2011]a)Glycol with KOHb)Zn-Hg with HClc)Li Al H4d)H2 and Pt as catalystCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Clemmensen reduction of a ketone is carried outin the presence of which of the following ?[2011]a)Glycol with KOHb)Zn-Hg with HClc)Li Al H4d)H2 and Pt as catalystCorrect answer is option 'B'. Can you explain this answer?.
Clemmensen reduction of a ketone is carried outin the presence of which of the following ?[2011]a)Glycol with KOHb)Zn-Hg with HClc)Li Al H4d)H2 and Pt as catalystCorrect answer is option 'B'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Clemmensen reduction of a ketone is carried outin the presence of which of the following ?[2011]a)Glycol with KOHb)Zn-Hg with HClc)Li Al H4d)H2 and Pt as catalystCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Clemmensen reduction of a ketone is carried outin the presence of which of the following ?[2011]a)Glycol with KOHb)Zn-Hg with HClc)Li Al H4d)H2 and Pt as catalystCorrect answer is option 'B'. Can you explain this answer?.
Solutions for Clemmensen reduction of a ketone is carried outin the presence of which of the following ?[2011]a)Glycol with KOHb)Zn-Hg with HClc)Li Al H4d)H2 and Pt as catalystCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Clemmensen reduction of a ketone is carried outin the presence of which of the following ?[2011]a)Glycol with KOHb)Zn-Hg with HClc)Li Al H4d)H2 and Pt as catalystCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Clemmensen reduction of a ketone is carried outin the presence of which of the following ?[2011]a)Glycol with KOHb)Zn-Hg with HClc)Li Al H4d)H2 and Pt as catalystCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for Clemmensen reduction of a ketone is carried outin the presence of which of the following ?[2011]a)Glycol with KOHb)Zn-Hg with HClc)Li Al H4d)H2 and Pt as catalystCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of Clemmensen reduction of a ketone is carried outin the presence of which of the following ?[2011]a)Glycol with KOHb)Zn-Hg with HClc)Li Al H4d)H2 and Pt as catalystCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Clemmensen reduction of a ketone is carried outin the presence of which of the following ?[2011]a)Glycol with KOHb)Zn-Hg with HClc)Li Al H4d)H2 and Pt as catalystCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























