Class 12 Exam > Class 12 Questions > Which of the following is correct ? [2001]a)O...
Start Learning for Free
Which of the following is correct ? [2001]
- a)On reduction of any aldehyde, secondary alcohol is formed
- b)Reaction of vegetable oil with H2SO4 gives glycerine
- c)Sucrose on reaction with NaCl gives invert sugar
- d)Alcoholic iodine gives iodoform with NaOH
Correct answer is option 'D'. Can you explain this answer?
Verified Answer
Which of the following is correct ? [2001]a)On reduction of any aldehy...
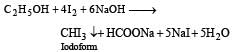
Most Upvoted Answer
Which of the following is correct ? [2001]a)On reduction of any aldehy...
The other three are wrong as
Option a) On reduction of any aldehyde,primary alcohol is formed.
Option b) Reaction of vegetable oils with H2SO4 gives unsaturated alcohol.
Option c ) Sucrose on reaction with NaCl gives no product.[No reaction].
Option a) On reduction of any aldehyde,primary alcohol is formed.
Option b) Reaction of vegetable oils with H2SO4 gives unsaturated alcohol.
Option c ) Sucrose on reaction with NaCl gives no product.[No reaction].
Free Test
FREE
| Start Free Test |
Community Answer
Which of the following is correct ? [2001]a)On reduction of any aldehy...
The correct answer is option 'D': Alcoholic iodine gives iodoform with NaOH.
Explanation:
Iodoform is a yellow crystalline solid that has a distinct odor and is commonly used as an antiseptic. It is also a useful reagent in organic chemistry for the identification of methyl ketones.
When an alcohol reacts with an oxidizing agent such as iodine in the presence of a base like sodium hydroxide (NaOH), it undergoes oxidation to form an aldehyde or ketone. In the case of iodoform formation, the alcohol being used is tertiary alcohol.
Here is the stepwise reaction for the formation of iodoform from a tertiary alcohol using alcoholic iodine and NaOH:
1. The reaction starts with the deprotonation of the alcohol by the base (NaOH) to form the alkoxide ion.
2. The alkoxide ion then reacts with iodine (I2) to form the iodoalkoxide intermediate.
3. The iodoalkoxide undergoes nucleophilic substitution with iodine ions (I-) present in the reaction mixture to form the triiodomethoxide ion.
4. The triiodomethoxide ion then loses a hydroxide ion (OH-) to form iodoform (CHI3) and the base (NaOH) is regenerated.
The overall reaction can be represented as follows:
R3COH + 4I2 + 6NaOH → R3CCI3 + 5NaI + 5H2O
In this reaction, R represents an alkyl group in the tertiary alcohol.
It is important to note that this reaction is specific to tertiary alcohols. Primary and secondary alcohols do not undergo this reaction to form iodoform. Instead, they undergo oxidation to form aldehydes and ketones, respectively.
In conclusion, option 'D' is the correct answer because alcoholic iodine (I2) reacts with NaOH to give iodoform (CHI3) when a tertiary alcohol is used as the starting material.
Explanation:
Iodoform is a yellow crystalline solid that has a distinct odor and is commonly used as an antiseptic. It is also a useful reagent in organic chemistry for the identification of methyl ketones.
When an alcohol reacts with an oxidizing agent such as iodine in the presence of a base like sodium hydroxide (NaOH), it undergoes oxidation to form an aldehyde or ketone. In the case of iodoform formation, the alcohol being used is tertiary alcohol.
Here is the stepwise reaction for the formation of iodoform from a tertiary alcohol using alcoholic iodine and NaOH:
1. The reaction starts with the deprotonation of the alcohol by the base (NaOH) to form the alkoxide ion.
2. The alkoxide ion then reacts with iodine (I2) to form the iodoalkoxide intermediate.
3. The iodoalkoxide undergoes nucleophilic substitution with iodine ions (I-) present in the reaction mixture to form the triiodomethoxide ion.
4. The triiodomethoxide ion then loses a hydroxide ion (OH-) to form iodoform (CHI3) and the base (NaOH) is regenerated.
The overall reaction can be represented as follows:
R3COH + 4I2 + 6NaOH → R3CCI3 + 5NaI + 5H2O
In this reaction, R represents an alkyl group in the tertiary alcohol.
It is important to note that this reaction is specific to tertiary alcohols. Primary and secondary alcohols do not undergo this reaction to form iodoform. Instead, they undergo oxidation to form aldehydes and ketones, respectively.
In conclusion, option 'D' is the correct answer because alcoholic iodine (I2) reacts with NaOH to give iodoform (CHI3) when a tertiary alcohol is used as the starting material.

|
Explore Courses for Class 12 exam
|

|
Question Description
Which of the following is correct ? [2001]a)On reduction of any aldehyde, secondary alcohol is formedb)Reaction of vegetable oil with H2SO4 gives glycerinec)Sucrose on reaction with NaCl gives invert sugard)Alcoholic iodine gives iodoform with NaOHCorrect answer is option 'D'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Which of the following is correct ? [2001]a)On reduction of any aldehyde, secondary alcohol is formedb)Reaction of vegetable oil with H2SO4 gives glycerinec)Sucrose on reaction with NaCl gives invert sugard)Alcoholic iodine gives iodoform with NaOHCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following is correct ? [2001]a)On reduction of any aldehyde, secondary alcohol is formedb)Reaction of vegetable oil with H2SO4 gives glycerinec)Sucrose on reaction with NaCl gives invert sugard)Alcoholic iodine gives iodoform with NaOHCorrect answer is option 'D'. Can you explain this answer?.
Which of the following is correct ? [2001]a)On reduction of any aldehyde, secondary alcohol is formedb)Reaction of vegetable oil with H2SO4 gives glycerinec)Sucrose on reaction with NaCl gives invert sugard)Alcoholic iodine gives iodoform with NaOHCorrect answer is option 'D'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Which of the following is correct ? [2001]a)On reduction of any aldehyde, secondary alcohol is formedb)Reaction of vegetable oil with H2SO4 gives glycerinec)Sucrose on reaction with NaCl gives invert sugard)Alcoholic iodine gives iodoform with NaOHCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following is correct ? [2001]a)On reduction of any aldehyde, secondary alcohol is formedb)Reaction of vegetable oil with H2SO4 gives glycerinec)Sucrose on reaction with NaCl gives invert sugard)Alcoholic iodine gives iodoform with NaOHCorrect answer is option 'D'. Can you explain this answer?.
Solutions for Which of the following is correct ? [2001]a)On reduction of any aldehyde, secondary alcohol is formedb)Reaction of vegetable oil with H2SO4 gives glycerinec)Sucrose on reaction with NaCl gives invert sugard)Alcoholic iodine gives iodoform with NaOHCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Which of the following is correct ? [2001]a)On reduction of any aldehyde, secondary alcohol is formedb)Reaction of vegetable oil with H2SO4 gives glycerinec)Sucrose on reaction with NaCl gives invert sugard)Alcoholic iodine gives iodoform with NaOHCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which of the following is correct ? [2001]a)On reduction of any aldehyde, secondary alcohol is formedb)Reaction of vegetable oil with H2SO4 gives glycerinec)Sucrose on reaction with NaCl gives invert sugard)Alcoholic iodine gives iodoform with NaOHCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for Which of the following is correct ? [2001]a)On reduction of any aldehyde, secondary alcohol is formedb)Reaction of vegetable oil with H2SO4 gives glycerinec)Sucrose on reaction with NaCl gives invert sugard)Alcoholic iodine gives iodoform with NaOHCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of Which of the following is correct ? [2001]a)On reduction of any aldehyde, secondary alcohol is formedb)Reaction of vegetable oil with H2SO4 gives glycerinec)Sucrose on reaction with NaCl gives invert sugard)Alcoholic iodine gives iodoform with NaOHCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which of the following is correct ? [2001]a)On reduction of any aldehyde, secondary alcohol is formedb)Reaction of vegetable oil with H2SO4 gives glycerinec)Sucrose on reaction with NaCl gives invert sugard)Alcoholic iodine gives iodoform with NaOHCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















