Class 11 Exam > Class 11 Questions > When 5 litres of a gas mixture of methane and...
Start Learning for Free
When 5 litres of a gas mixture of methane and propane is perfectly combusted at 0°C and 1 atmosphere, 16 litre of oxygen at the same temperature and pressure is consumed. The amount of heat released from this combustion in kJ (ΔHcomb (CH4) = 890 kJ mol–1,
ΔHcomb (C3H8) = 2220 kJ mol–1) is [NEET Kar. 2013]
ΔHcomb (C3H8) = 2220 kJ mol–1) is [NEET Kar. 2013]
- a)32
- b)38
- c)317
- d)477
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
When 5 litres of a gas mixture of methane and propane is perfectly com...
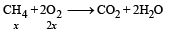
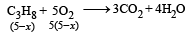
2x + 5(5– x) = 16
⇒ x = 3L
∴ Heat released
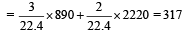
Most Upvoted Answer
When 5 litres of a gas mixture of methane and propane is perfectly com...
°C and 1 atm pressure, the volume of the resulting products is 18.85 L. The composition of the gas mixture can be determined using the ideal gas law, which states:
PV = nRT
Where P is the pressure, V is the volume, n is the number of moles of gas, R is the gas constant, and T is the temperature in Kelvin.
Assuming that the combustion reaction is complete and that the products are carbon dioxide and water, the balanced chemical equation is:
CH4 + 2C3H8 → 5CO2 + 8H2O
From the equation, it can be seen that the number of moles of carbon dioxide produced is equal to the number of moles of methane and propane consumed. Let x be the number of moles of methane in the mixture and y be the number of moles of propane. Then:
x + y = n (where n is the total number of moles of gas in the mixture)
From the ideal gas law, the number of moles of gas can be calculated as:
n = PV/RT
Substituting the values given in the problem, we get:
n = (1 atm)(5 L)/(0.0821 L-atm/mol-K)(273 K) = 0.22 mol
Now, we can set up a system of equations to solve for x and y:
x + y = 0.22 (from the conservation of moles)
5x + 8y = PV/RT (from the balanced chemical equation and ideal gas law)
Substituting the given values, we get:
5x + 8y = (1 atm)(18.85 L)/(0.0821 L-atm/mol-K)(273 K)
Simplifying the equation, we get:
5x + 8y = 1.99
Multiplying the first equation by 8 and subtracting it from the second equation, we get:
5x + 8y - 1.76 = 0
Solving for y in terms of x, we get:
y = -5x/8 + 0.22/8
Simplifying the equation, we get:
y = -0.625x + 0.0275
Substituting this equation into the first equation, we get:
x + (-0.625x + 0.0275) = 0.22
Solving for x, we get:
x = 0.1 mol (approx.)
Substituting this value into the equation for y, we get:
y = 0.035 mol (approx.)
Therefore, the gas mixture contains approximately 0.1 mol of methane and 0.035 mol of propane. The mole fraction of methane in the mixture is:
x/n = 0.1/0.22 = 0.45
And the mole fraction of propane is:
y/n = 0.035/0.22 = 0.16
The remaining fraction of the mixture is made up of other gases, such as nitrogen and oxygen.
PV = nRT
Where P is the pressure, V is the volume, n is the number of moles of gas, R is the gas constant, and T is the temperature in Kelvin.
Assuming that the combustion reaction is complete and that the products are carbon dioxide and water, the balanced chemical equation is:
CH4 + 2C3H8 → 5CO2 + 8H2O
From the equation, it can be seen that the number of moles of carbon dioxide produced is equal to the number of moles of methane and propane consumed. Let x be the number of moles of methane in the mixture and y be the number of moles of propane. Then:
x + y = n (where n is the total number of moles of gas in the mixture)
From the ideal gas law, the number of moles of gas can be calculated as:
n = PV/RT
Substituting the values given in the problem, we get:
n = (1 atm)(5 L)/(0.0821 L-atm/mol-K)(273 K) = 0.22 mol
Now, we can set up a system of equations to solve for x and y:
x + y = 0.22 (from the conservation of moles)
5x + 8y = PV/RT (from the balanced chemical equation and ideal gas law)
Substituting the given values, we get:
5x + 8y = (1 atm)(18.85 L)/(0.0821 L-atm/mol-K)(273 K)
Simplifying the equation, we get:
5x + 8y = 1.99
Multiplying the first equation by 8 and subtracting it from the second equation, we get:
5x + 8y - 1.76 = 0
Solving for y in terms of x, we get:
y = -5x/8 + 0.22/8
Simplifying the equation, we get:
y = -0.625x + 0.0275
Substituting this equation into the first equation, we get:
x + (-0.625x + 0.0275) = 0.22
Solving for x, we get:
x = 0.1 mol (approx.)
Substituting this value into the equation for y, we get:
y = 0.035 mol (approx.)
Therefore, the gas mixture contains approximately 0.1 mol of methane and 0.035 mol of propane. The mole fraction of methane in the mixture is:
x/n = 0.1/0.22 = 0.45
And the mole fraction of propane is:
y/n = 0.035/0.22 = 0.16
The remaining fraction of the mixture is made up of other gases, such as nitrogen and oxygen.

|
Explore Courses for Class 11 exam
|

|
Question Description
When 5 litres of a gas mixture of methane and propane is perfectly combusted at 0°C and 1 atmosphere, 16 litre of oxygen at the same temperature and pressure is consumed. The amount of heat released from this combustion in kJ (ΔHcomb (CH4) = 890 kJ mol–1,ΔHcomb (C3H8) = 2220 kJ mol–1) is [NEET Kar. 2013]a)32b)38c)317d)477Correct answer is option 'C'. Can you explain this answer? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about When 5 litres of a gas mixture of methane and propane is perfectly combusted at 0°C and 1 atmosphere, 16 litre of oxygen at the same temperature and pressure is consumed. The amount of heat released from this combustion in kJ (ΔHcomb (CH4) = 890 kJ mol–1,ΔHcomb (C3H8) = 2220 kJ mol–1) is [NEET Kar. 2013]a)32b)38c)317d)477Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for When 5 litres of a gas mixture of methane and propane is perfectly combusted at 0°C and 1 atmosphere, 16 litre of oxygen at the same temperature and pressure is consumed. The amount of heat released from this combustion in kJ (ΔHcomb (CH4) = 890 kJ mol–1,ΔHcomb (C3H8) = 2220 kJ mol–1) is [NEET Kar. 2013]a)32b)38c)317d)477Correct answer is option 'C'. Can you explain this answer?.
When 5 litres of a gas mixture of methane and propane is perfectly combusted at 0°C and 1 atmosphere, 16 litre of oxygen at the same temperature and pressure is consumed. The amount of heat released from this combustion in kJ (ΔHcomb (CH4) = 890 kJ mol–1,ΔHcomb (C3H8) = 2220 kJ mol–1) is [NEET Kar. 2013]a)32b)38c)317d)477Correct answer is option 'C'. Can you explain this answer? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about When 5 litres of a gas mixture of methane and propane is perfectly combusted at 0°C and 1 atmosphere, 16 litre of oxygen at the same temperature and pressure is consumed. The amount of heat released from this combustion in kJ (ΔHcomb (CH4) = 890 kJ mol–1,ΔHcomb (C3H8) = 2220 kJ mol–1) is [NEET Kar. 2013]a)32b)38c)317d)477Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for When 5 litres of a gas mixture of methane and propane is perfectly combusted at 0°C and 1 atmosphere, 16 litre of oxygen at the same temperature and pressure is consumed. The amount of heat released from this combustion in kJ (ΔHcomb (CH4) = 890 kJ mol–1,ΔHcomb (C3H8) = 2220 kJ mol–1) is [NEET Kar. 2013]a)32b)38c)317d)477Correct answer is option 'C'. Can you explain this answer?.
Solutions for When 5 litres of a gas mixture of methane and propane is perfectly combusted at 0°C and 1 atmosphere, 16 litre of oxygen at the same temperature and pressure is consumed. The amount of heat released from this combustion in kJ (ΔHcomb (CH4) = 890 kJ mol–1,ΔHcomb (C3H8) = 2220 kJ mol–1) is [NEET Kar. 2013]a)32b)38c)317d)477Correct answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of When 5 litres of a gas mixture of methane and propane is perfectly combusted at 0°C and 1 atmosphere, 16 litre of oxygen at the same temperature and pressure is consumed. The amount of heat released from this combustion in kJ (ΔHcomb (CH4) = 890 kJ mol–1,ΔHcomb (C3H8) = 2220 kJ mol–1) is [NEET Kar. 2013]a)32b)38c)317d)477Correct answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
When 5 litres of a gas mixture of methane and propane is perfectly combusted at 0°C and 1 atmosphere, 16 litre of oxygen at the same temperature and pressure is consumed. The amount of heat released from this combustion in kJ (ΔHcomb (CH4) = 890 kJ mol–1,ΔHcomb (C3H8) = 2220 kJ mol–1) is [NEET Kar. 2013]a)32b)38c)317d)477Correct answer is option 'C'. Can you explain this answer?, a detailed solution for When 5 litres of a gas mixture of methane and propane is perfectly combusted at 0°C and 1 atmosphere, 16 litre of oxygen at the same temperature and pressure is consumed. The amount of heat released from this combustion in kJ (ΔHcomb (CH4) = 890 kJ mol–1,ΔHcomb (C3H8) = 2220 kJ mol–1) is [NEET Kar. 2013]a)32b)38c)317d)477Correct answer is option 'C'. Can you explain this answer? has been provided alongside types of When 5 litres of a gas mixture of methane and propane is perfectly combusted at 0°C and 1 atmosphere, 16 litre of oxygen at the same temperature and pressure is consumed. The amount of heat released from this combustion in kJ (ΔHcomb (CH4) = 890 kJ mol–1,ΔHcomb (C3H8) = 2220 kJ mol–1) is [NEET Kar. 2013]a)32b)38c)317d)477Correct answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice When 5 litres of a gas mixture of methane and propane is perfectly combusted at 0°C and 1 atmosphere, 16 litre of oxygen at the same temperature and pressure is consumed. The amount of heat released from this combustion in kJ (ΔHcomb (CH4) = 890 kJ mol–1,ΔHcomb (C3H8) = 2220 kJ mol–1) is [NEET Kar. 2013]a)32b)38c)317d)477Correct answer is option 'C'. Can you explain this answer? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























