Class 11 Exam > Class 11 Questions > The electronegativity difference between N an...
Start Learning for Free
The electronegativity difference between N and F is greater than that between N and H yet the dipole moment of NH3 (1.5 D) is larger than that of NF3 (0.2D). This is because [2006]
- a)in NH3 the atomic dipole and bond dipole are in the same direction whereas in NF3 these are in opposite directions
- b)in NH3 as well as NF3 the atomic dipole and bond dipole are in opposite directions
- c)in NH3 the atomic dipole and bond dipole are in the opposite directions whereas in NF3 these are in the same direction
- d)in NH3 as well as in NF3 the atomic dipole and bond dipole are in the same direction
Correct answer is option 'A'. Can you explain this answer?
Verified Answer
The electronegativity difference between N and F is greater than that ...
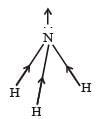
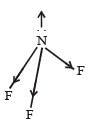
In NH3 the atomic dipole and bond dipole are in the same direction whereas in NF3 these are in opposite direction so in the former case they are added up whereas in the latter case net result is reduction of dipole moment. It has been shown in the following figure :
Most Upvoted Answer
The electronegativity difference between N and F is greater than that ...
Electronegativity Difference
- The electronegativity difference between two atoms in a molecule is a measure of the inequality of sharing of electrons between them.
- In the case of NH3 (ammonia), the electronegativity difference between nitrogen (N) and hydrogen (H) is relatively small, but still greater than that between nitrogen (N) and fluorine (F).
- This is because fluorine is more electronegative than hydrogen, resulting in a larger electronegativity difference between N and F compared to N and H.
Dipole Moment
- The dipole moment of a molecule is a measure of the overall polarity of the molecule.
- It is determined by the magnitude and direction of the individual bond moments within the molecule.
- In the case of NH3, the dipole moment is 1.5 D, which indicates that the molecule is polar.
- In the case of NF3, the dipole moment is 0.2 D, which also indicates that the molecule is polar but to a lesser extent.
Explanation of the Correct Answer (Option A)
- In NH3, the atomic dipole (due to the electronegativity difference between N and H) and the bond dipole (due to the geometry of the molecule) are in the same direction.
- This means that the individual bond moments reinforce each other, resulting in a larger overall dipole moment.
- In NF3, the atomic dipole (due to the electronegativity difference between N and F) and the bond dipole (due to the geometry of the molecule) are in opposite directions.
- This means that the individual bond moments partially cancel each other out, resulting in a smaller overall dipole moment.
Other Options Explanation
- Option B states that in both NH3 and NF3, the atomic dipole and bond dipole are in opposite directions. This is incorrect because the atomic dipole and bond dipole in NH3 are in the same direction.
- Option C states that in NH3, the atomic dipole and bond dipole are in opposite directions, whereas in NF3, they are in the same direction. This is incorrect because in NH3, the atomic dipole and bond dipole are in the same direction.
- Option D states that in both NH3 and NF3, the atomic dipole and bond dipole are in the same direction. This is incorrect because in NF3, the atomic dipole and bond dipole are in opposite directions.
Therefore, the correct answer is option A, where the atomic dipole and bond dipole in NH3 are in the same direction, resulting in a larger dipole moment compared to NF3.
- The electronegativity difference between two atoms in a molecule is a measure of the inequality of sharing of electrons between them.
- In the case of NH3 (ammonia), the electronegativity difference between nitrogen (N) and hydrogen (H) is relatively small, but still greater than that between nitrogen (N) and fluorine (F).
- This is because fluorine is more electronegative than hydrogen, resulting in a larger electronegativity difference between N and F compared to N and H.
Dipole Moment
- The dipole moment of a molecule is a measure of the overall polarity of the molecule.
- It is determined by the magnitude and direction of the individual bond moments within the molecule.
- In the case of NH3, the dipole moment is 1.5 D, which indicates that the molecule is polar.
- In the case of NF3, the dipole moment is 0.2 D, which also indicates that the molecule is polar but to a lesser extent.
Explanation of the Correct Answer (Option A)
- In NH3, the atomic dipole (due to the electronegativity difference between N and H) and the bond dipole (due to the geometry of the molecule) are in the same direction.
- This means that the individual bond moments reinforce each other, resulting in a larger overall dipole moment.
- In NF3, the atomic dipole (due to the electronegativity difference between N and F) and the bond dipole (due to the geometry of the molecule) are in opposite directions.
- This means that the individual bond moments partially cancel each other out, resulting in a smaller overall dipole moment.
Other Options Explanation
- Option B states that in both NH3 and NF3, the atomic dipole and bond dipole are in opposite directions. This is incorrect because the atomic dipole and bond dipole in NH3 are in the same direction.
- Option C states that in NH3, the atomic dipole and bond dipole are in opposite directions, whereas in NF3, they are in the same direction. This is incorrect because in NH3, the atomic dipole and bond dipole are in the same direction.
- Option D states that in both NH3 and NF3, the atomic dipole and bond dipole are in the same direction. This is incorrect because in NF3, the atomic dipole and bond dipole are in opposite directions.
Therefore, the correct answer is option A, where the atomic dipole and bond dipole in NH3 are in the same direction, resulting in a larger dipole moment compared to NF3.

|
Explore Courses for Class 11 exam
|

|
Question Description
The electronegativity difference between N and F is greater than that between N and H yet the dipole moment of NH3 (1.5 D) is larger than that of NF3 (0.2D). This is because [2006]a)in NH3 the atomic dipole and bond dipole are in the same direction whereas in NF3 these are in opposite directionsb)in NH3 as well as NF3 the atomic dipole and bond dipole are in opposite directionsc)in NH3 the atomic dipole and bond dipole are in the opposite directions whereas in NF3 these are in the same directiond)in NH3 as well as in NF3 the atomic dipole and bond dipole are in the same directionCorrect answer is option 'A'. Can you explain this answer? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about The electronegativity difference between N and F is greater than that between N and H yet the dipole moment of NH3 (1.5 D) is larger than that of NF3 (0.2D). This is because [2006]a)in NH3 the atomic dipole and bond dipole are in the same direction whereas in NF3 these are in opposite directionsb)in NH3 as well as NF3 the atomic dipole and bond dipole are in opposite directionsc)in NH3 the atomic dipole and bond dipole are in the opposite directions whereas in NF3 these are in the same directiond)in NH3 as well as in NF3 the atomic dipole and bond dipole are in the same directionCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The electronegativity difference between N and F is greater than that between N and H yet the dipole moment of NH3 (1.5 D) is larger than that of NF3 (0.2D). This is because [2006]a)in NH3 the atomic dipole and bond dipole are in the same direction whereas in NF3 these are in opposite directionsb)in NH3 as well as NF3 the atomic dipole and bond dipole are in opposite directionsc)in NH3 the atomic dipole and bond dipole are in the opposite directions whereas in NF3 these are in the same directiond)in NH3 as well as in NF3 the atomic dipole and bond dipole are in the same directionCorrect answer is option 'A'. Can you explain this answer?.
The electronegativity difference between N and F is greater than that between N and H yet the dipole moment of NH3 (1.5 D) is larger than that of NF3 (0.2D). This is because [2006]a)in NH3 the atomic dipole and bond dipole are in the same direction whereas in NF3 these are in opposite directionsb)in NH3 as well as NF3 the atomic dipole and bond dipole are in opposite directionsc)in NH3 the atomic dipole and bond dipole are in the opposite directions whereas in NF3 these are in the same directiond)in NH3 as well as in NF3 the atomic dipole and bond dipole are in the same directionCorrect answer is option 'A'. Can you explain this answer? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about The electronegativity difference between N and F is greater than that between N and H yet the dipole moment of NH3 (1.5 D) is larger than that of NF3 (0.2D). This is because [2006]a)in NH3 the atomic dipole and bond dipole are in the same direction whereas in NF3 these are in opposite directionsb)in NH3 as well as NF3 the atomic dipole and bond dipole are in opposite directionsc)in NH3 the atomic dipole and bond dipole are in the opposite directions whereas in NF3 these are in the same directiond)in NH3 as well as in NF3 the atomic dipole and bond dipole are in the same directionCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The electronegativity difference between N and F is greater than that between N and H yet the dipole moment of NH3 (1.5 D) is larger than that of NF3 (0.2D). This is because [2006]a)in NH3 the atomic dipole and bond dipole are in the same direction whereas in NF3 these are in opposite directionsb)in NH3 as well as NF3 the atomic dipole and bond dipole are in opposite directionsc)in NH3 the atomic dipole and bond dipole are in the opposite directions whereas in NF3 these are in the same directiond)in NH3 as well as in NF3 the atomic dipole and bond dipole are in the same directionCorrect answer is option 'A'. Can you explain this answer?.
Solutions for The electronegativity difference between N and F is greater than that between N and H yet the dipole moment of NH3 (1.5 D) is larger than that of NF3 (0.2D). This is because [2006]a)in NH3 the atomic dipole and bond dipole are in the same direction whereas in NF3 these are in opposite directionsb)in NH3 as well as NF3 the atomic dipole and bond dipole are in opposite directionsc)in NH3 the atomic dipole and bond dipole are in the opposite directions whereas in NF3 these are in the same directiond)in NH3 as well as in NF3 the atomic dipole and bond dipole are in the same directionCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of The electronegativity difference between N and F is greater than that between N and H yet the dipole moment of NH3 (1.5 D) is larger than that of NF3 (0.2D). This is because [2006]a)in NH3 the atomic dipole and bond dipole are in the same direction whereas in NF3 these are in opposite directionsb)in NH3 as well as NF3 the atomic dipole and bond dipole are in opposite directionsc)in NH3 the atomic dipole and bond dipole are in the opposite directions whereas in NF3 these are in the same directiond)in NH3 as well as in NF3 the atomic dipole and bond dipole are in the same directionCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The electronegativity difference between N and F is greater than that between N and H yet the dipole moment of NH3 (1.5 D) is larger than that of NF3 (0.2D). This is because [2006]a)in NH3 the atomic dipole and bond dipole are in the same direction whereas in NF3 these are in opposite directionsb)in NH3 as well as NF3 the atomic dipole and bond dipole are in opposite directionsc)in NH3 the atomic dipole and bond dipole are in the opposite directions whereas in NF3 these are in the same directiond)in NH3 as well as in NF3 the atomic dipole and bond dipole are in the same directionCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for The electronegativity difference between N and F is greater than that between N and H yet the dipole moment of NH3 (1.5 D) is larger than that of NF3 (0.2D). This is because [2006]a)in NH3 the atomic dipole and bond dipole are in the same direction whereas in NF3 these are in opposite directionsb)in NH3 as well as NF3 the atomic dipole and bond dipole are in opposite directionsc)in NH3 the atomic dipole and bond dipole are in the opposite directions whereas in NF3 these are in the same directiond)in NH3 as well as in NF3 the atomic dipole and bond dipole are in the same directionCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of The electronegativity difference between N and F is greater than that between N and H yet the dipole moment of NH3 (1.5 D) is larger than that of NF3 (0.2D). This is because [2006]a)in NH3 the atomic dipole and bond dipole are in the same direction whereas in NF3 these are in opposite directionsb)in NH3 as well as NF3 the atomic dipole and bond dipole are in opposite directionsc)in NH3 the atomic dipole and bond dipole are in the opposite directions whereas in NF3 these are in the same directiond)in NH3 as well as in NF3 the atomic dipole and bond dipole are in the same directionCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The electronegativity difference between N and F is greater than that between N and H yet the dipole moment of NH3 (1.5 D) is larger than that of NF3 (0.2D). This is because [2006]a)in NH3 the atomic dipole and bond dipole are in the same direction whereas in NF3 these are in opposite directionsb)in NH3 as well as NF3 the atomic dipole and bond dipole are in opposite directionsc)in NH3 the atomic dipole and bond dipole are in the opposite directions whereas in NF3 these are in the same directiond)in NH3 as well as in NF3 the atomic dipole and bond dipole are in the same directionCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















