Class 11 Exam > Class 11 Questions > Which one of the following molecules will for...
Start Learning for Free
Which one of the following molecules will form a linear polymeric structure due to hydrogen bonding? [2000]
- a)NH3
- b)H2O
- c)HCl
- d)HF
Correct answer is option 'D'. Can you explain this answer?
Verified Answer
Which one of the following molecules will form a linear polymeric stru...
In the case of HCl we can see that hydrogen is bonded to chlorine atoms so it will not show hydrogen bonding.
HF:
Here we can see that HF show a linear polymeric structure due to hydrogen bonding.
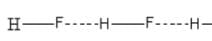
Here we can see that though H2O show polymerisation due to hydrogen bonding it is not linear polymerisation.
HF:
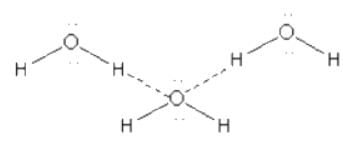
Here we can see that HF show a linear polymeric structure due to hydrogen bonding.
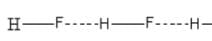
Here we can see that though H2O show polymerisation due to hydrogen bonding it is not linear polymerisation.

NH3
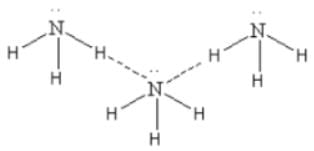

Here we can see that though NH3 show polymerisation due to hydrogen bonding it is not linear polymerisation.
Thus, the correct option is (B) HF.
Thus, the correct option is (B) HF.
Most Upvoted Answer
Which one of the following molecules will form a linear polymeric stru...
Explanation:
Hydrogen bonding occurs when a hydrogen atom is bonded to a highly electronegative atom such as nitrogen (N), oxygen (O), or fluorine (F). This type of bonding is a special type of dipole-dipole interaction where the hydrogen atom acts as a bridge between two electronegative atoms.
In the given options, only HF (hydrogen fluoride) contains a hydrogen atom bonded to a highly electronegative atom (fluorine), making it capable of forming hydrogen bonds.
Hydrogen bonding in HF:
- In HF, the hydrogen atom is bonded to fluorine, which is highly electronegative.
- The fluorine atom attracts the electron pair in the H-F bond, creating a partial positive charge on the hydrogen atom and a partial negative charge on the fluorine atom.
- This partial positive charge on the hydrogen atom can then form a hydrogen bond with another electronegative atom, such as another HF molecule.
- The hydrogen bond is formed between the partial positive charge on the hydrogen atom of one HF molecule and the partial negative charge on the fluorine atom of another HF molecule.
Linear polymeric structure:
- In the case of HF, the hydrogen bonds can form between multiple HF molecules, resulting in the formation of a linear polymeric structure.
- The hydrogen bonds act as bridges between the HF molecules, connecting them in a linear chain-like structure.
Other options:
- NH3 (ammonia), H2O (water), and HCl (hydrochloric acid) do not contain a hydrogen atom bonded to a highly electronegative atom, so they cannot form hydrogen bonds.
- NH3 and HCl can form dipole-dipole interactions, but these interactions are not strong enough to result in the formation of a linear polymeric structure.
- H2O can form hydrogen bonds, but the arrangement of the hydrogen bonds in water molecules does not lead to the formation of a linear polymeric structure.
Therefore, the molecule that will form a linear polymeric structure due to hydrogen bonding is HF.
Hydrogen bonding occurs when a hydrogen atom is bonded to a highly electronegative atom such as nitrogen (N), oxygen (O), or fluorine (F). This type of bonding is a special type of dipole-dipole interaction where the hydrogen atom acts as a bridge between two electronegative atoms.
In the given options, only HF (hydrogen fluoride) contains a hydrogen atom bonded to a highly electronegative atom (fluorine), making it capable of forming hydrogen bonds.
Hydrogen bonding in HF:
- In HF, the hydrogen atom is bonded to fluorine, which is highly electronegative.
- The fluorine atom attracts the electron pair in the H-F bond, creating a partial positive charge on the hydrogen atom and a partial negative charge on the fluorine atom.
- This partial positive charge on the hydrogen atom can then form a hydrogen bond with another electronegative atom, such as another HF molecule.
- The hydrogen bond is formed between the partial positive charge on the hydrogen atom of one HF molecule and the partial negative charge on the fluorine atom of another HF molecule.
Linear polymeric structure:
- In the case of HF, the hydrogen bonds can form between multiple HF molecules, resulting in the formation of a linear polymeric structure.
- The hydrogen bonds act as bridges between the HF molecules, connecting them in a linear chain-like structure.
Other options:
- NH3 (ammonia), H2O (water), and HCl (hydrochloric acid) do not contain a hydrogen atom bonded to a highly electronegative atom, so they cannot form hydrogen bonds.
- NH3 and HCl can form dipole-dipole interactions, but these interactions are not strong enough to result in the formation of a linear polymeric structure.
- H2O can form hydrogen bonds, but the arrangement of the hydrogen bonds in water molecules does not lead to the formation of a linear polymeric structure.
Therefore, the molecule that will form a linear polymeric structure due to hydrogen bonding is HF.

|
Explore Courses for Class 11 exam
|

|
Question Description
Which one of the following molecules will form a linear polymeric structure due to hydrogen bonding? [2000]a)NH3b)H2Oc)HCld)HFCorrect answer is option 'D'. Can you explain this answer? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Which one of the following molecules will form a linear polymeric structure due to hydrogen bonding? [2000]a)NH3b)H2Oc)HCld)HFCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which one of the following molecules will form a linear polymeric structure due to hydrogen bonding? [2000]a)NH3b)H2Oc)HCld)HFCorrect answer is option 'D'. Can you explain this answer?.
Which one of the following molecules will form a linear polymeric structure due to hydrogen bonding? [2000]a)NH3b)H2Oc)HCld)HFCorrect answer is option 'D'. Can you explain this answer? for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about Which one of the following molecules will form a linear polymeric structure due to hydrogen bonding? [2000]a)NH3b)H2Oc)HCld)HFCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which one of the following molecules will form a linear polymeric structure due to hydrogen bonding? [2000]a)NH3b)H2Oc)HCld)HFCorrect answer is option 'D'. Can you explain this answer?.
Solutions for Which one of the following molecules will form a linear polymeric structure due to hydrogen bonding? [2000]a)NH3b)H2Oc)HCld)HFCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of Which one of the following molecules will form a linear polymeric structure due to hydrogen bonding? [2000]a)NH3b)H2Oc)HCld)HFCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which one of the following molecules will form a linear polymeric structure due to hydrogen bonding? [2000]a)NH3b)H2Oc)HCld)HFCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for Which one of the following molecules will form a linear polymeric structure due to hydrogen bonding? [2000]a)NH3b)H2Oc)HCld)HFCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of Which one of the following molecules will form a linear polymeric structure due to hydrogen bonding? [2000]a)NH3b)H2Oc)HCld)HFCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which one of the following molecules will form a linear polymeric structure due to hydrogen bonding? [2000]a)NH3b)H2Oc)HCld)HFCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















