Class 9 Exam > Class 9 Questions > Hòw can we seaptrate two immiscible liquids?
Start Learning for Free
Hòw can we seaptrate two immiscible liquids?
Verified Answer
Hòw can we seaptrate two immiscible liquids?
Mixtures of liquids can be separated according to their properties. The technique used depends on whether the liquids dissolve in each other, and so are miscible, or if they are immiscible. Fractional distillation is a technique used to separate liquids according to their boiling points. Chromatography is used to separate mixtures of coloured compounds.
Separation of liquids
Liquids can be described in two ways – immiscible and miscible. The separation technique used for each liquid depends on the properties of the liquids.
Immiscible liquids
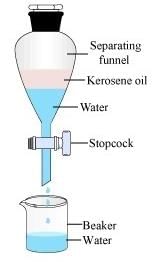
Oil and water can be separated using a funnel
Immiscible means that the liquids don't dissolve in each other – oil and water are an example. It is possible to shake up the liquids and get them to mix but they soon separate. Separating immiscible liquids is done simply using a separating funnel. The two liquids are put into the funnel and are left for a short time to settle out and form two layers. The tap of the funnel is opened and the bottom liquid is allowed to run. The two liquids are now separate.

 This question is part of UPSC exam. View all Class 9 courses
This question is part of UPSC exam. View all Class 9 courses
Most Upvoted Answer
Hòw can we seaptrate two immiscible liquids?
Separating immiscible liquids is done simply using a separatingfunnel. The two liquids are put into the funnel and are left for a short time to settle out and formtwo layers. The tap of the funnel is opened and the bottom liquidis allowed to run. The two liquidsare now separate.
Community Answer
Hòw can we seaptrate two immiscible liquids?
Separation of Immiscible Liquids
Separating two immiscible liquids can be achieved through various methods based on the physical properties of the liquids involved. Immiscible liquids are those that do not mix together and form distinct layers when combined. Here are some common techniques used for their separation:
1. Gravity Separation:
- Gravity separation is the simplest and most commonly used method for separating immiscible liquids.
- It relies on the difference in density between the two liquids to allow them to separate naturally.
- The liquids are placed in a container and allowed to settle over time, with the denser liquid settling at the bottom and the lighter liquid forming a distinct layer on top.
- Once the separation is complete, the liquids can be carefully decanted or siphoned off.
2. Separating Funnel:
- A separating funnel is a specialized apparatus used for separating immiscible liquids with different densities.
- It consists of a conical shape with a stopcock at the bottom for easy drainage.
- The liquids are poured into the funnel and allowed to settle. The denser liquid can then be drained out by opening the stopcock at the bottom, while the lighter liquid remains in the funnel.
- This method is particularly useful when the liquids have a significant density difference.
3. Centrifugation:
- Centrifugation is a technique that uses centrifugal force to separate immiscible liquids.
- The mixture is placed in a centrifuge, which spins rapidly, generating a strong force that pushes the denser liquid to the bottom.
- After centrifugation, the liquids form distinct layers, and the denser liquid can be carefully removed using a pipette or a syringe.
4. Distillation:
- Distillation is a method used when the immiscible liquids have different boiling points.
- The mixture is heated, and the liquid with the lower boiling point evaporates first.
- The vapors are then condensed and collected, resulting in the separation of the two liquids.
5. Solvent Extraction:
- Solvent extraction is a technique used when the immiscible liquids are soluble in different solvents.
- A suitable solvent is added to the mixture, which selectively dissolves one of the liquids.
- The dissolved liquid can then be separated from the mixture by decantation or filtration.
6. Membrane Separation:
- Membrane separation involves the use of a semi-permeable membrane that allows the passage of one liquid while blocking the other.
- The mixture is passed through the membrane, with one liquid permeating through and the other being retained.
- This method is commonly used in industries for large-scale separation processes.
7. Adsorption:
- Adsorption is a technique where one liquid is selectively adsorbed onto a solid material, separating it from the other liquid.
- The mixture is passed through a bed of adsorbent material, which traps one liquid while allowing the other to pass through.
- The trapped liquid can then be recovered by desorption or washing the adsorbent material.
These methods provide effective ways to separate immiscible liquids based on their physical properties such as density, solubility, or boiling points. The choice of the appropriate method depends on the specific characteristics of the liquids and the desired outcome of the separation process.
Separating two immiscible liquids can be achieved through various methods based on the physical properties of the liquids involved. Immiscible liquids are those that do not mix together and form distinct layers when combined. Here are some common techniques used for their separation:
1. Gravity Separation:
- Gravity separation is the simplest and most commonly used method for separating immiscible liquids.
- It relies on the difference in density between the two liquids to allow them to separate naturally.
- The liquids are placed in a container and allowed to settle over time, with the denser liquid settling at the bottom and the lighter liquid forming a distinct layer on top.
- Once the separation is complete, the liquids can be carefully decanted or siphoned off.
2. Separating Funnel:
- A separating funnel is a specialized apparatus used for separating immiscible liquids with different densities.
- It consists of a conical shape with a stopcock at the bottom for easy drainage.
- The liquids are poured into the funnel and allowed to settle. The denser liquid can then be drained out by opening the stopcock at the bottom, while the lighter liquid remains in the funnel.
- This method is particularly useful when the liquids have a significant density difference.
3. Centrifugation:
- Centrifugation is a technique that uses centrifugal force to separate immiscible liquids.
- The mixture is placed in a centrifuge, which spins rapidly, generating a strong force that pushes the denser liquid to the bottom.
- After centrifugation, the liquids form distinct layers, and the denser liquid can be carefully removed using a pipette or a syringe.
4. Distillation:
- Distillation is a method used when the immiscible liquids have different boiling points.
- The mixture is heated, and the liquid with the lower boiling point evaporates first.
- The vapors are then condensed and collected, resulting in the separation of the two liquids.
5. Solvent Extraction:
- Solvent extraction is a technique used when the immiscible liquids are soluble in different solvents.
- A suitable solvent is added to the mixture, which selectively dissolves one of the liquids.
- The dissolved liquid can then be separated from the mixture by decantation or filtration.
6. Membrane Separation:
- Membrane separation involves the use of a semi-permeable membrane that allows the passage of one liquid while blocking the other.
- The mixture is passed through the membrane, with one liquid permeating through and the other being retained.
- This method is commonly used in industries for large-scale separation processes.
7. Adsorption:
- Adsorption is a technique where one liquid is selectively adsorbed onto a solid material, separating it from the other liquid.
- The mixture is passed through a bed of adsorbent material, which traps one liquid while allowing the other to pass through.
- The trapped liquid can then be recovered by desorption or washing the adsorbent material.
These methods provide effective ways to separate immiscible liquids based on their physical properties such as density, solubility, or boiling points. The choice of the appropriate method depends on the specific characteristics of the liquids and the desired outcome of the separation process.

|
Explore Courses for Class 9 exam
|

|
Similar Class 9 Doubts
Hòw can we seaptrate two immiscible liquids?
Question Description
Hòw can we seaptrate two immiscible liquids? for Class 9 2025 is part of Class 9 preparation. The Question and answers have been prepared according to the Class 9 exam syllabus. Information about Hòw can we seaptrate two immiscible liquids? covers all topics & solutions for Class 9 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Hòw can we seaptrate two immiscible liquids?.
Hòw can we seaptrate two immiscible liquids? for Class 9 2025 is part of Class 9 preparation. The Question and answers have been prepared according to the Class 9 exam syllabus. Information about Hòw can we seaptrate two immiscible liquids? covers all topics & solutions for Class 9 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Hòw can we seaptrate two immiscible liquids?.
Solutions for Hòw can we seaptrate two immiscible liquids? in English & in Hindi are available as part of our courses for Class 9.
Download more important topics, notes, lectures and mock test series for Class 9 Exam by signing up for free.
Here you can find the meaning of Hòw can we seaptrate two immiscible liquids? defined & explained in the simplest way possible. Besides giving the explanation of
Hòw can we seaptrate two immiscible liquids?, a detailed solution for Hòw can we seaptrate two immiscible liquids? has been provided alongside types of Hòw can we seaptrate two immiscible liquids? theory, EduRev gives you an
ample number of questions to practice Hòw can we seaptrate two immiscible liquids? tests, examples and also practice Class 9 tests.

|
Explore Courses for Class 9 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


























