Chemistry Exam > Chemistry Questions > On heating anhydrous Na2CO3.........is evolve...
Start Learning for Free
On heating anhydrous Na2CO3.........is evolved:
- a)CO2
- b)Water vapour
- c)CO
- d)No gas
Correct answer is option 'D'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
On heating anhydrous Na2CO3.........is evolved:a)CO2b)Water vapourc)CO...
Ans.
Na2CO3 is sodium carbonate. Like most metal carbonates it undergoes thermal decomposition to produce carbon dioxide. Here's the equation:
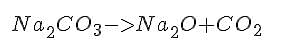
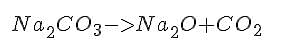
Sodium carbonate absorbs water to form a decahydrate, meaning that each molecule is associated with 10 water molecules. As it's heated it begins to dehydrate, or lose water molecules to form the anhydrous compound. The anhydride begins to gradually decompose to CO2 and Na2O at a temperature near its melting point of 851�C. However, it can be melted. Molten sodium carbonate is used as flux in glass production.
Most Upvoted Answer
On heating anhydrous Na2CO3.........is evolved:a)CO2b)Water vapourc)CO...
Explanation:
Anhydrous Na2CO3 is a white powder that is used in various industrial processes, such as the production of glass, soaps, and detergents. When heated, this compound undergoes a chemical change, but it does not produce any gas. The reason for this is that anhydrous Na2CO3 does not contain any water molecules, which are typically the source of gas evolution during thermal decomposition.
The thermal decomposition of Na2CO3 is a process that involves the breaking of chemical bonds within the compound. At high temperatures, the energy input causes the bonds between the sodium and carbonate ions to weaken and eventually break. However, since there are no water molecules present, there is no gas produced during this process.
Conclusion:
In conclusion, the correct answer to this question is option 'D', which states that no gas is evolved when anhydrous Na2CO3 is heated. This is because anhydrous Na2CO3 does not contain any water molecules, which are typically the source of gas evolution during thermal decomposition.
Anhydrous Na2CO3 is a white powder that is used in various industrial processes, such as the production of glass, soaps, and detergents. When heated, this compound undergoes a chemical change, but it does not produce any gas. The reason for this is that anhydrous Na2CO3 does not contain any water molecules, which are typically the source of gas evolution during thermal decomposition.
The thermal decomposition of Na2CO3 is a process that involves the breaking of chemical bonds within the compound. At high temperatures, the energy input causes the bonds between the sodium and carbonate ions to weaken and eventually break. However, since there are no water molecules present, there is no gas produced during this process.
Conclusion:
In conclusion, the correct answer to this question is option 'D', which states that no gas is evolved when anhydrous Na2CO3 is heated. This is because anhydrous Na2CO3 does not contain any water molecules, which are typically the source of gas evolution during thermal decomposition.

|
Explore Courses for Chemistry exam
|

|
Similar Chemistry Doubts
On heating anhydrous Na2CO3.........is evolved:a)CO2b)Water vapourc)COd)No gasCorrect answer is option 'D'. Can you explain this answer?
Question Description
On heating anhydrous Na2CO3.........is evolved:a)CO2b)Water vapourc)COd)No gasCorrect answer is option 'D'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about On heating anhydrous Na2CO3.........is evolved:a)CO2b)Water vapourc)COd)No gasCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for On heating anhydrous Na2CO3.........is evolved:a)CO2b)Water vapourc)COd)No gasCorrect answer is option 'D'. Can you explain this answer?.
On heating anhydrous Na2CO3.........is evolved:a)CO2b)Water vapourc)COd)No gasCorrect answer is option 'D'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about On heating anhydrous Na2CO3.........is evolved:a)CO2b)Water vapourc)COd)No gasCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for On heating anhydrous Na2CO3.........is evolved:a)CO2b)Water vapourc)COd)No gasCorrect answer is option 'D'. Can you explain this answer?.
Solutions for On heating anhydrous Na2CO3.........is evolved:a)CO2b)Water vapourc)COd)No gasCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of On heating anhydrous Na2CO3.........is evolved:a)CO2b)Water vapourc)COd)No gasCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
On heating anhydrous Na2CO3.........is evolved:a)CO2b)Water vapourc)COd)No gasCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for On heating anhydrous Na2CO3.........is evolved:a)CO2b)Water vapourc)COd)No gasCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of On heating anhydrous Na2CO3.........is evolved:a)CO2b)Water vapourc)COd)No gasCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice On heating anhydrous Na2CO3.........is evolved:a)CO2b)Water vapourc)COd)No gasCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















