Class 12 Exam > Class 12 Questions > Which of the following oxides will be the lea...
Start Learning for Free
Which of the following oxides will be the least acidic?[1996]
- a)As4O6
- b)As4O10
- c)P4O10
- d)P4O6
Correct answer is option 'A'. Can you explain this answer?
Verified Answer
Which of the following oxides will be the least acidic?[1996]a)As4O6b)...
As th e O.N of the central atom of the compounds increases acidic strength of that compound also increases and on moving from top to bottom in groups acidic strength of oxides also decrease due to decreasing electronegativity in groups.
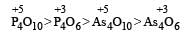
Most Upvoted Answer
Which of the following oxides will be the least acidic?[1996]a)As4O6b)...
Explanation:
To determine the acidity of oxides, we need to analyze their chemical formula and structure.
As4O6:
This oxide contains arsenic (As) and oxygen (O) atoms. It has 4 arsenic atoms and 6 oxygen atoms. The chemical formula suggests that each arsenic atom is bonded to 1.5 oxygen atoms on average. This indicates that the oxide has a lower oxygen-to-arsenic ratio compared to As4O10.
As4O10:
This oxide also contains arsenic (As) and oxygen (O) atoms. It has 4 arsenic atoms and 10 oxygen atoms. The chemical formula suggests that each arsenic atom is bonded to 2.5 oxygen atoms on average. This indicates that the oxide has a higher oxygen-to-arsenic ratio compared to As4O6.
P4O10:
This oxide contains phosphorus (P) and oxygen (O) atoms. It has 4 phosphorus atoms and 10 oxygen atoms. The chemical formula suggests that each phosphorus atom is bonded to 2.5 oxygen atoms on average. This oxide has a similar oxygen-to-phosphorus ratio as As4O10.
P4O6:
This oxide also contains phosphorus (P) and oxygen (O) atoms. It has 4 phosphorus atoms and 6 oxygen atoms. The chemical formula suggests that each phosphorus atom is bonded to 1.5 oxygen atoms on average. This oxide has a lower oxygen-to-phosphorus ratio compared to P4O10.
Analysis:
The acidity of oxides is influenced by their ability to donate protons (H+) in aqueous solutions. Generally, oxides with a higher oxygen-to-metal ratio are more acidic because they can readily release protons.
Comparing the oxygen-to-metal ratios of the given oxides:
- As4O6 has the lowest oxygen-to-arsenic ratio (1.5:1)
- As4O10 and P4O10 have the highest oxygen-to-metal ratios (2.5:1)
- P4O6 has an intermediate oxygen-to-phosphorus ratio (1.5:1)
Conclusion:
As4O6 has the lowest oxygen-to-metal ratio among the given oxides, indicating that it is the least acidic. Therefore, option A is the correct answer.
To determine the acidity of oxides, we need to analyze their chemical formula and structure.
As4O6:
This oxide contains arsenic (As) and oxygen (O) atoms. It has 4 arsenic atoms and 6 oxygen atoms. The chemical formula suggests that each arsenic atom is bonded to 1.5 oxygen atoms on average. This indicates that the oxide has a lower oxygen-to-arsenic ratio compared to As4O10.
As4O10:
This oxide also contains arsenic (As) and oxygen (O) atoms. It has 4 arsenic atoms and 10 oxygen atoms. The chemical formula suggests that each arsenic atom is bonded to 2.5 oxygen atoms on average. This indicates that the oxide has a higher oxygen-to-arsenic ratio compared to As4O6.
P4O10:
This oxide contains phosphorus (P) and oxygen (O) atoms. It has 4 phosphorus atoms and 10 oxygen atoms. The chemical formula suggests that each phosphorus atom is bonded to 2.5 oxygen atoms on average. This oxide has a similar oxygen-to-phosphorus ratio as As4O10.
P4O6:
This oxide also contains phosphorus (P) and oxygen (O) atoms. It has 4 phosphorus atoms and 6 oxygen atoms. The chemical formula suggests that each phosphorus atom is bonded to 1.5 oxygen atoms on average. This oxide has a lower oxygen-to-phosphorus ratio compared to P4O10.
Analysis:
The acidity of oxides is influenced by their ability to donate protons (H+) in aqueous solutions. Generally, oxides with a higher oxygen-to-metal ratio are more acidic because they can readily release protons.
Comparing the oxygen-to-metal ratios of the given oxides:
- As4O6 has the lowest oxygen-to-arsenic ratio (1.5:1)
- As4O10 and P4O10 have the highest oxygen-to-metal ratios (2.5:1)
- P4O6 has an intermediate oxygen-to-phosphorus ratio (1.5:1)
Conclusion:
As4O6 has the lowest oxygen-to-metal ratio among the given oxides, indicating that it is the least acidic. Therefore, option A is the correct answer.

|
Explore Courses for Class 12 exam
|

|
Question Description
Which of the following oxides will be the least acidic?[1996]a)As4O6b)As4O10c)P4O10d)P4O6Correct answer is option 'A'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Which of the following oxides will be the least acidic?[1996]a)As4O6b)As4O10c)P4O10d)P4O6Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following oxides will be the least acidic?[1996]a)As4O6b)As4O10c)P4O10d)P4O6Correct answer is option 'A'. Can you explain this answer?.
Which of the following oxides will be the least acidic?[1996]a)As4O6b)As4O10c)P4O10d)P4O6Correct answer is option 'A'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Which of the following oxides will be the least acidic?[1996]a)As4O6b)As4O10c)P4O10d)P4O6Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following oxides will be the least acidic?[1996]a)As4O6b)As4O10c)P4O10d)P4O6Correct answer is option 'A'. Can you explain this answer?.
Solutions for Which of the following oxides will be the least acidic?[1996]a)As4O6b)As4O10c)P4O10d)P4O6Correct answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Which of the following oxides will be the least acidic?[1996]a)As4O6b)As4O10c)P4O10d)P4O6Correct answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which of the following oxides will be the least acidic?[1996]a)As4O6b)As4O10c)P4O10d)P4O6Correct answer is option 'A'. Can you explain this answer?, a detailed solution for Which of the following oxides will be the least acidic?[1996]a)As4O6b)As4O10c)P4O10d)P4O6Correct answer is option 'A'. Can you explain this answer? has been provided alongside types of Which of the following oxides will be the least acidic?[1996]a)As4O6b)As4O10c)P4O10d)P4O6Correct answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which of the following oxides will be the least acidic?[1996]a)As4O6b)As4O10c)P4O10d)P4O6Correct answer is option 'A'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















