Class 12 Exam > Class 12 Questions > Polyanion formation is maximum in [1994]a)Nit...
Start Learning for Free
Polyanion formation is maximum in [1994]
- a)Nitrogen
- b)Oxygen
- c)Sulphur
- d)Boron
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
Polyanion formation is maximum in [1994]a)Nitrogenb)Oxygenc)Sulphurd)B...
Due to greater tendency for catenation, sulphur shows property of polyanion formation to a greater extent. For example, in polysulphides such as 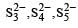
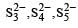
Most Upvoted Answer
Polyanion formation is maximum in [1994]a)Nitrogenb)Oxygenc)Sulphurd)B...
Polyanion formation is maximum in Sulphur.
Polyanions are negatively charged ions that contain more than one atom. The formation of polyanions depends on various factors such as the electronegativity of the atom, the number of valence electrons, and the ability to gain electrons to achieve a stable electron configuration.
Sulphur, with the atomic number 16, belongs to Group 16 (Group 6A) of the periodic table. It has six valence electrons in its outermost energy level. To achieve a stable electron configuration, sulphur needs to gain two electrons.
Here are the reasons why polyanion formation is maximum in Sulphur:
1. Electron configuration: Sulphur has six valence electrons in its outer energy level, and it needs to gain two electrons to achieve a stable electron configuration of the nearest noble gas, argon. This makes it easier for sulphur to gain electrons and form negatively charged ions.
2. Electronegativity: Sulphur has a moderate electronegativity, which means it has a tendency to attract electrons towards itself. This property is favorable for the formation of polyanions as it allows sulphur to gain electrons and become negatively charged.
3. Size of the atom: Sulphur is a relatively large atom compared to other elements in Group 16. The larger the atom, the more easily it can accommodate additional electrons and form stable polyanions.
4. Stability of polyanions: Sulphur can form a variety of stable polyanions, such as sulfide ions (S2-), bisulfide ions (HS-), and thiosulfate ions (S2O3^2-). These polyanions have strong bonds and are stable in various chemical reactions.
In contrast, other options like Nitrogen, Oxygen, and Boron do not have the same combination of electron configuration, electronegativity, and size that make polyanion formation as favorable as Sulphur.
In conclusion, Sulphur exhibits maximum polyanion formation due to its electron configuration, moderate electronegativity, ability to accommodate additional electrons, and the stability of the formed polyanions.
Polyanions are negatively charged ions that contain more than one atom. The formation of polyanions depends on various factors such as the electronegativity of the atom, the number of valence electrons, and the ability to gain electrons to achieve a stable electron configuration.
Sulphur, with the atomic number 16, belongs to Group 16 (Group 6A) of the periodic table. It has six valence electrons in its outermost energy level. To achieve a stable electron configuration, sulphur needs to gain two electrons.
Here are the reasons why polyanion formation is maximum in Sulphur:
1. Electron configuration: Sulphur has six valence electrons in its outer energy level, and it needs to gain two electrons to achieve a stable electron configuration of the nearest noble gas, argon. This makes it easier for sulphur to gain electrons and form negatively charged ions.
2. Electronegativity: Sulphur has a moderate electronegativity, which means it has a tendency to attract electrons towards itself. This property is favorable for the formation of polyanions as it allows sulphur to gain electrons and become negatively charged.
3. Size of the atom: Sulphur is a relatively large atom compared to other elements in Group 16. The larger the atom, the more easily it can accommodate additional electrons and form stable polyanions.
4. Stability of polyanions: Sulphur can form a variety of stable polyanions, such as sulfide ions (S2-), bisulfide ions (HS-), and thiosulfate ions (S2O3^2-). These polyanions have strong bonds and are stable in various chemical reactions.
In contrast, other options like Nitrogen, Oxygen, and Boron do not have the same combination of electron configuration, electronegativity, and size that make polyanion formation as favorable as Sulphur.
In conclusion, Sulphur exhibits maximum polyanion formation due to its electron configuration, moderate electronegativity, ability to accommodate additional electrons, and the stability of the formed polyanions.

|
Explore Courses for Class 12 exam
|

|
Question Description
Polyanion formation is maximum in [1994]a)Nitrogenb)Oxygenc)Sulphurd)BoronCorrect answer is option 'C'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Polyanion formation is maximum in [1994]a)Nitrogenb)Oxygenc)Sulphurd)BoronCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Polyanion formation is maximum in [1994]a)Nitrogenb)Oxygenc)Sulphurd)BoronCorrect answer is option 'C'. Can you explain this answer?.
Polyanion formation is maximum in [1994]a)Nitrogenb)Oxygenc)Sulphurd)BoronCorrect answer is option 'C'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Polyanion formation is maximum in [1994]a)Nitrogenb)Oxygenc)Sulphurd)BoronCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Polyanion formation is maximum in [1994]a)Nitrogenb)Oxygenc)Sulphurd)BoronCorrect answer is option 'C'. Can you explain this answer?.
Solutions for Polyanion formation is maximum in [1994]a)Nitrogenb)Oxygenc)Sulphurd)BoronCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Polyanion formation is maximum in [1994]a)Nitrogenb)Oxygenc)Sulphurd)BoronCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Polyanion formation is maximum in [1994]a)Nitrogenb)Oxygenc)Sulphurd)BoronCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for Polyanion formation is maximum in [1994]a)Nitrogenb)Oxygenc)Sulphurd)BoronCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of Polyanion formation is maximum in [1994]a)Nitrogenb)Oxygenc)Sulphurd)BoronCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Polyanion formation is maximum in [1994]a)Nitrogenb)Oxygenc)Sulphurd)BoronCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























