Class 12 Exam > Class 12 Questions > The bleaching action of chlorine is due to [1...
Start Learning for Free
The bleaching action of chlorine is due to [1991]
- a)Reduction
- b)Hydrogenation
- c)Chlorination
- d)Oxidation
Correct answer is option 'D'. Can you explain this answer?
Verified Answer
The bleaching action of chlorine is due to [1991]a)Reductionb)Hydrogen...
Bleaching action of chlorine is due to oxidation in presence of moisture. It is permanent
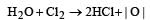
Colouring matter + | O | → colourless matter
Most Upvoted Answer
The bleaching action of chlorine is due to [1991]a)Reductionb)Hydrogen...
The correct answer is option 'D': Oxidation.
Explanation:
Chlorine is a highly reactive element that has a strong oxidizing property. The bleaching action of chlorine is due to its ability to oxidize colored compounds present in the material being bleached. This process involves the transfer of electrons from the colored compounds to chlorine atoms, resulting in the removal of color.
Chlorine acts as an oxidizing agent by accepting electrons from other substances and becoming reduced in the process. In the case of bleaching, chlorine oxidizes the colored compounds by accepting electrons from them, thereby reducing itself.
Chlorine's ability to oxidize is due to its high electronegativity and the presence of an empty 3p orbital in its electron configuration. The electronegativity of an element determines its tendency to attract electrons towards itself in a chemical reaction. Chlorine's high electronegativity allows it to attract electrons from other substances.
The empty 3p orbital in chlorine's electron configuration allows it to accommodate the electrons donated by the colored compounds during the oxidation process. This leads to the formation of chloride ions (Cl-) as chlorine gains electrons.
The oxidation of colored compounds by chlorine results in the breaking of double bonds and conjugated systems, which are responsible for the absorption of visible light and the production of color. The removal of these conjugated systems through oxidation leads to the decolorization or bleaching effect.
In summary, the bleaching action of chlorine is due to its strong oxidizing property. Chlorine acts as an oxidizing agent by accepting electrons from colored compounds, causing them to lose their color. The ability of chlorine to oxidize is a result of its high electronegativity and the presence of an empty 3p orbital in its electron configuration.
Explanation:
Chlorine is a highly reactive element that has a strong oxidizing property. The bleaching action of chlorine is due to its ability to oxidize colored compounds present in the material being bleached. This process involves the transfer of electrons from the colored compounds to chlorine atoms, resulting in the removal of color.
Chlorine acts as an oxidizing agent by accepting electrons from other substances and becoming reduced in the process. In the case of bleaching, chlorine oxidizes the colored compounds by accepting electrons from them, thereby reducing itself.
Chlorine's ability to oxidize is due to its high electronegativity and the presence of an empty 3p orbital in its electron configuration. The electronegativity of an element determines its tendency to attract electrons towards itself in a chemical reaction. Chlorine's high electronegativity allows it to attract electrons from other substances.
The empty 3p orbital in chlorine's electron configuration allows it to accommodate the electrons donated by the colored compounds during the oxidation process. This leads to the formation of chloride ions (Cl-) as chlorine gains electrons.
The oxidation of colored compounds by chlorine results in the breaking of double bonds and conjugated systems, which are responsible for the absorption of visible light and the production of color. The removal of these conjugated systems through oxidation leads to the decolorization or bleaching effect.
In summary, the bleaching action of chlorine is due to its strong oxidizing property. Chlorine acts as an oxidizing agent by accepting electrons from colored compounds, causing them to lose their color. The ability of chlorine to oxidize is a result of its high electronegativity and the presence of an empty 3p orbital in its electron configuration.

|
Explore Courses for Class 12 exam
|

|
Question Description
The bleaching action of chlorine is due to [1991]a)Reductionb)Hydrogenationc)Chlorinationd)OxidationCorrect answer is option 'D'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about The bleaching action of chlorine is due to [1991]a)Reductionb)Hydrogenationc)Chlorinationd)OxidationCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The bleaching action of chlorine is due to [1991]a)Reductionb)Hydrogenationc)Chlorinationd)OxidationCorrect answer is option 'D'. Can you explain this answer?.
The bleaching action of chlorine is due to [1991]a)Reductionb)Hydrogenationc)Chlorinationd)OxidationCorrect answer is option 'D'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about The bleaching action of chlorine is due to [1991]a)Reductionb)Hydrogenationc)Chlorinationd)OxidationCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The bleaching action of chlorine is due to [1991]a)Reductionb)Hydrogenationc)Chlorinationd)OxidationCorrect answer is option 'D'. Can you explain this answer?.
Solutions for The bleaching action of chlorine is due to [1991]a)Reductionb)Hydrogenationc)Chlorinationd)OxidationCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of The bleaching action of chlorine is due to [1991]a)Reductionb)Hydrogenationc)Chlorinationd)OxidationCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The bleaching action of chlorine is due to [1991]a)Reductionb)Hydrogenationc)Chlorinationd)OxidationCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for The bleaching action of chlorine is due to [1991]a)Reductionb)Hydrogenationc)Chlorinationd)OxidationCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of The bleaching action of chlorine is due to [1991]a)Reductionb)Hydrogenationc)Chlorinationd)OxidationCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The bleaching action of chlorine is due to [1991]a)Reductionb)Hydrogenationc)Chlorinationd)OxidationCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















