Class 12 Exam > Class 12 Questions > At 25°C molar conductance of 0.1 molar aq...
Start Learning for Free
At 25°C molar conductance of 0.1 molar aqueous solution of ammonium hydroxide is 9.54 ohm-1 cm2mol-1 and at infinite dilution its molar conductance is 238 ohm-1 cm2 mol-1. The degree or ionisation of ammonium hydroxide at the same concentration and temperature is :[NEET 2013]
- a)20.800%
- b)4.008%
- c)40.800%
- d)2.080%
Correct answer is option 'B'. Can you explain this answer?
Verified Answer
At 25°C molar conductance of 0.1 molar aqueous solution of ammoniu...
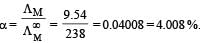
Most Upvoted Answer
At 25°C molar conductance of 0.1 molar aqueous solution of ammoniu...
At 25 years old, many individuals are starting to establish their careers and may be in entry-level positions in their chosen fields. They may also be pursuing further education such as graduate degrees or certifications to advance their career prospects. Additionally, many people at this age may be starting to think about long-term goals such as homeownership or starting a family. It is a time of exploration and self-discovery, as individuals are still learning about themselves and figuring out their place in the world. Socially, many 25-year-olds are still actively involved in their friend groups and may be dating or in a committed relationship. Overall, 25 is a transitional age where individuals are navigating the early stages of adulthood and working towards building a foundation for their future.

|
Explore Courses for Class 12 exam
|

|
Question Description
At 25°C molar conductance of 0.1 molar aqueous solution of ammonium hydroxide is 9.54 ohm-1 cm2mol-1 and at infinite dilution its molar conductance is 238 ohm-1 cm2 mol-1. The degree or ionisation of ammonium hydroxide at the same concentration and temperature is :[NEET 2013]a)20.800%b)4.008%c)40.800%d)2.080%Correct answer is option 'B'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about At 25°C molar conductance of 0.1 molar aqueous solution of ammonium hydroxide is 9.54 ohm-1 cm2mol-1 and at infinite dilution its molar conductance is 238 ohm-1 cm2 mol-1. The degree or ionisation of ammonium hydroxide at the same concentration and temperature is :[NEET 2013]a)20.800%b)4.008%c)40.800%d)2.080%Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for At 25°C molar conductance of 0.1 molar aqueous solution of ammonium hydroxide is 9.54 ohm-1 cm2mol-1 and at infinite dilution its molar conductance is 238 ohm-1 cm2 mol-1. The degree or ionisation of ammonium hydroxide at the same concentration and temperature is :[NEET 2013]a)20.800%b)4.008%c)40.800%d)2.080%Correct answer is option 'B'. Can you explain this answer?.
At 25°C molar conductance of 0.1 molar aqueous solution of ammonium hydroxide is 9.54 ohm-1 cm2mol-1 and at infinite dilution its molar conductance is 238 ohm-1 cm2 mol-1. The degree or ionisation of ammonium hydroxide at the same concentration and temperature is :[NEET 2013]a)20.800%b)4.008%c)40.800%d)2.080%Correct answer is option 'B'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about At 25°C molar conductance of 0.1 molar aqueous solution of ammonium hydroxide is 9.54 ohm-1 cm2mol-1 and at infinite dilution its molar conductance is 238 ohm-1 cm2 mol-1. The degree or ionisation of ammonium hydroxide at the same concentration and temperature is :[NEET 2013]a)20.800%b)4.008%c)40.800%d)2.080%Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for At 25°C molar conductance of 0.1 molar aqueous solution of ammonium hydroxide is 9.54 ohm-1 cm2mol-1 and at infinite dilution its molar conductance is 238 ohm-1 cm2 mol-1. The degree or ionisation of ammonium hydroxide at the same concentration and temperature is :[NEET 2013]a)20.800%b)4.008%c)40.800%d)2.080%Correct answer is option 'B'. Can you explain this answer?.
Solutions for At 25°C molar conductance of 0.1 molar aqueous solution of ammonium hydroxide is 9.54 ohm-1 cm2mol-1 and at infinite dilution its molar conductance is 238 ohm-1 cm2 mol-1. The degree or ionisation of ammonium hydroxide at the same concentration and temperature is :[NEET 2013]a)20.800%b)4.008%c)40.800%d)2.080%Correct answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of At 25°C molar conductance of 0.1 molar aqueous solution of ammonium hydroxide is 9.54 ohm-1 cm2mol-1 and at infinite dilution its molar conductance is 238 ohm-1 cm2 mol-1. The degree or ionisation of ammonium hydroxide at the same concentration and temperature is :[NEET 2013]a)20.800%b)4.008%c)40.800%d)2.080%Correct answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
At 25°C molar conductance of 0.1 molar aqueous solution of ammonium hydroxide is 9.54 ohm-1 cm2mol-1 and at infinite dilution its molar conductance is 238 ohm-1 cm2 mol-1. The degree or ionisation of ammonium hydroxide at the same concentration and temperature is :[NEET 2013]a)20.800%b)4.008%c)40.800%d)2.080%Correct answer is option 'B'. Can you explain this answer?, a detailed solution for At 25°C molar conductance of 0.1 molar aqueous solution of ammonium hydroxide is 9.54 ohm-1 cm2mol-1 and at infinite dilution its molar conductance is 238 ohm-1 cm2 mol-1. The degree or ionisation of ammonium hydroxide at the same concentration and temperature is :[NEET 2013]a)20.800%b)4.008%c)40.800%d)2.080%Correct answer is option 'B'. Can you explain this answer? has been provided alongside types of At 25°C molar conductance of 0.1 molar aqueous solution of ammonium hydroxide is 9.54 ohm-1 cm2mol-1 and at infinite dilution its molar conductance is 238 ohm-1 cm2 mol-1. The degree or ionisation of ammonium hydroxide at the same concentration and temperature is :[NEET 2013]a)20.800%b)4.008%c)40.800%d)2.080%Correct answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice At 25°C molar conductance of 0.1 molar aqueous solution of ammonium hydroxide is 9.54 ohm-1 cm2mol-1 and at infinite dilution its molar conductance is 238 ohm-1 cm2 mol-1. The degree or ionisation of ammonium hydroxide at the same concentration and temperature is :[NEET 2013]a)20.800%b)4.008%c)40.800%d)2.080%Correct answer is option 'B'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















