Class 12 Exam > Class 12 Questions > An electr och emical cell is set up as: Pt; H...
Start Learning for Free
An electr och emical cell is set up as: Pt; H2 (1atm)|HCl(0.1 M) || CH3COOH (0.1 M)| H2 (1atm); Pt. The e.m.f of this cell will not be zero, because [1995]
- a)the temperature is constant
- b)e.m.f depends on molarities of acids used
- c)acids used in two compartments are different
- d)pH of 0.1 M HCl and 0.1 M CH3COOH is not same
Correct answer is option 'D'. Can you explain this answer?
Verified Answer
An electr och emical cell is set up as: Pt; H2 (1atm)|HCl(0.1 M) || CH...
For a concentration cell having different concentrations of ions.
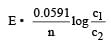
If all the concentrations are identical then obviously the cell voltage is zero. But as the pH of 0.1 M HCl (strong acid) & pH of 0.1M CH3 COOH is (weak acid) not same, therefore the cell voltage is not zero.
Most Upvoted Answer
An electr och emical cell is set up as: Pt; H2 (1atm)|HCl(0.1 M) || CH...
Explanation:
In an electrochemical cell, the emf (electromotive force) is the measure of the cell's ability to do work or drive an electric current through an external circuit. The emf of a cell is determined by a variety of factors, including the concentrations of the electrolytes used in the cell. In this specific setup, there are two compartments, each containing a different acid solution. Let's analyze each option to understand why option D is correct.
a) The temperature is constant:
The temperature of the cell does not have a direct impact on the emf. While temperature can affect the rate of the reaction, it does not change the concentrations of the electrolytes or the overall emf of the cell.
b) Emf depends on molarities of acids used:
This statement is partially correct. The emf of an electrochemical cell does depend on the concentrations of the electrolytes used in the cell. However, in this specific setup, both compartments have the same acid concentration (0.1 M). Therefore, the emf would not be affected solely based on the molarities of the acids.
c) Acids used in two compartments are different:
This statement is not correct. The acids used in both compartments are the same: HCl and CH3COOH. Therefore, the emf would not be affected by the difference in the acids used.
d) pH of 0.1 M HCl and 0.1 M CH3COOH is not the same:
This statement is correct and is the reason why the emf of the cell will not be zero. The pH of a solution is a measure of its acidity or alkalinity, which is determined by the concentration of hydrogen ions (H+). HCl is a strong acid and completely ionizes in water, resulting in a high concentration of H+ ions and a low pH. On the other hand, CH3COOH is a weak acid and only partially ionizes, resulting in a lower concentration of H+ ions and a higher pH compared to HCl. The difference in pH between the two compartments will result in a difference in the concentration of H+ ions, which will affect the overall emf of the cell.
Therefore, option D is the correct answer as the pH of 0.1 M HCl and 0.1 M CH3COOH is not the same, leading to a non-zero emf in the electrochemical cell.
In an electrochemical cell, the emf (electromotive force) is the measure of the cell's ability to do work or drive an electric current through an external circuit. The emf of a cell is determined by a variety of factors, including the concentrations of the electrolytes used in the cell. In this specific setup, there are two compartments, each containing a different acid solution. Let's analyze each option to understand why option D is correct.
a) The temperature is constant:
The temperature of the cell does not have a direct impact on the emf. While temperature can affect the rate of the reaction, it does not change the concentrations of the electrolytes or the overall emf of the cell.
b) Emf depends on molarities of acids used:
This statement is partially correct. The emf of an electrochemical cell does depend on the concentrations of the electrolytes used in the cell. However, in this specific setup, both compartments have the same acid concentration (0.1 M). Therefore, the emf would not be affected solely based on the molarities of the acids.
c) Acids used in two compartments are different:
This statement is not correct. The acids used in both compartments are the same: HCl and CH3COOH. Therefore, the emf would not be affected by the difference in the acids used.
d) pH of 0.1 M HCl and 0.1 M CH3COOH is not the same:
This statement is correct and is the reason why the emf of the cell will not be zero. The pH of a solution is a measure of its acidity or alkalinity, which is determined by the concentration of hydrogen ions (H+). HCl is a strong acid and completely ionizes in water, resulting in a high concentration of H+ ions and a low pH. On the other hand, CH3COOH is a weak acid and only partially ionizes, resulting in a lower concentration of H+ ions and a higher pH compared to HCl. The difference in pH between the two compartments will result in a difference in the concentration of H+ ions, which will affect the overall emf of the cell.
Therefore, option D is the correct answer as the pH of 0.1 M HCl and 0.1 M CH3COOH is not the same, leading to a non-zero emf in the electrochemical cell.

|
Explore Courses for Class 12 exam
|

|
Question Description
An electr och emical cell is set up as: Pt; H2 (1atm)|HCl(0.1 M) || CH3COOH (0.1 M)| H2 (1atm); Pt. The e.m.f of this cell will not be zero, because [1995]a)the temperature is constantb)e.m.f depends on molarities of acids usedc)acids used in two compartments are differentd)pH of 0.1 M HCl and 0.1 M CH3COOH is not sameCorrect answer is option 'D'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about An electr och emical cell is set up as: Pt; H2 (1atm)|HCl(0.1 M) || CH3COOH (0.1 M)| H2 (1atm); Pt. The e.m.f of this cell will not be zero, because [1995]a)the temperature is constantb)e.m.f depends on molarities of acids usedc)acids used in two compartments are differentd)pH of 0.1 M HCl and 0.1 M CH3COOH is not sameCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for An electr och emical cell is set up as: Pt; H2 (1atm)|HCl(0.1 M) || CH3COOH (0.1 M)| H2 (1atm); Pt. The e.m.f of this cell will not be zero, because [1995]a)the temperature is constantb)e.m.f depends on molarities of acids usedc)acids used in two compartments are differentd)pH of 0.1 M HCl and 0.1 M CH3COOH is not sameCorrect answer is option 'D'. Can you explain this answer?.
An electr och emical cell is set up as: Pt; H2 (1atm)|HCl(0.1 M) || CH3COOH (0.1 M)| H2 (1atm); Pt. The e.m.f of this cell will not be zero, because [1995]a)the temperature is constantb)e.m.f depends on molarities of acids usedc)acids used in two compartments are differentd)pH of 0.1 M HCl and 0.1 M CH3COOH is not sameCorrect answer is option 'D'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about An electr och emical cell is set up as: Pt; H2 (1atm)|HCl(0.1 M) || CH3COOH (0.1 M)| H2 (1atm); Pt. The e.m.f of this cell will not be zero, because [1995]a)the temperature is constantb)e.m.f depends on molarities of acids usedc)acids used in two compartments are differentd)pH of 0.1 M HCl and 0.1 M CH3COOH is not sameCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for An electr och emical cell is set up as: Pt; H2 (1atm)|HCl(0.1 M) || CH3COOH (0.1 M)| H2 (1atm); Pt. The e.m.f of this cell will not be zero, because [1995]a)the temperature is constantb)e.m.f depends on molarities of acids usedc)acids used in two compartments are differentd)pH of 0.1 M HCl and 0.1 M CH3COOH is not sameCorrect answer is option 'D'. Can you explain this answer?.
Solutions for An electr och emical cell is set up as: Pt; H2 (1atm)|HCl(0.1 M) || CH3COOH (0.1 M)| H2 (1atm); Pt. The e.m.f of this cell will not be zero, because [1995]a)the temperature is constantb)e.m.f depends on molarities of acids usedc)acids used in two compartments are differentd)pH of 0.1 M HCl and 0.1 M CH3COOH is not sameCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of An electr och emical cell is set up as: Pt; H2 (1atm)|HCl(0.1 M) || CH3COOH (0.1 M)| H2 (1atm); Pt. The e.m.f of this cell will not be zero, because [1995]a)the temperature is constantb)e.m.f depends on molarities of acids usedc)acids used in two compartments are differentd)pH of 0.1 M HCl and 0.1 M CH3COOH is not sameCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
An electr och emical cell is set up as: Pt; H2 (1atm)|HCl(0.1 M) || CH3COOH (0.1 M)| H2 (1atm); Pt. The e.m.f of this cell will not be zero, because [1995]a)the temperature is constantb)e.m.f depends on molarities of acids usedc)acids used in two compartments are differentd)pH of 0.1 M HCl and 0.1 M CH3COOH is not sameCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for An electr och emical cell is set up as: Pt; H2 (1atm)|HCl(0.1 M) || CH3COOH (0.1 M)| H2 (1atm); Pt. The e.m.f of this cell will not be zero, because [1995]a)the temperature is constantb)e.m.f depends on molarities of acids usedc)acids used in two compartments are differentd)pH of 0.1 M HCl and 0.1 M CH3COOH is not sameCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of An electr och emical cell is set up as: Pt; H2 (1atm)|HCl(0.1 M) || CH3COOH (0.1 M)| H2 (1atm); Pt. The e.m.f of this cell will not be zero, because [1995]a)the temperature is constantb)e.m.f depends on molarities of acids usedc)acids used in two compartments are differentd)pH of 0.1 M HCl and 0.1 M CH3COOH is not sameCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice An electr och emical cell is set up as: Pt; H2 (1atm)|HCl(0.1 M) || CH3COOH (0.1 M)| H2 (1atm); Pt. The e.m.f of this cell will not be zero, because [1995]a)the temperature is constantb)e.m.f depends on molarities of acids usedc)acids used in two compartments are differentd)pH of 0.1 M HCl and 0.1 M CH3COOH is not sameCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















