Civil Engineering (CE) Exam > Civil Engineering (CE) Questions > A city supply of 15000 cubic meters of water ...
Start Learning for Free
A city supply of 15000 cubic meters of water per day is treated with a chlorine dosage of 0.5 ppm. For this purpose, the requirement of 25% bleaching powder per day would be:
- a)300 kg
- b)75 kg
- c)30 kg
- d)750 kg
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
A city supply of 15000 cubic meters of water per day is treated with a...
Chlorine required for the city = Dose × Discharge
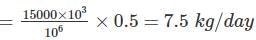
∴ Bleaching powder required 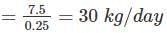
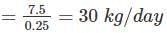
Most Upvoted Answer
A city supply of 15000 cubic meters of water per day is treated with a...
Given:
City supply of water per day = 15000 cubic meters
Chlorine dosage = 0.5 ppm
To find:
Requirement of 25% bleaching powder per day
Solution:
1. Calculation of chlorine required
Chlorine dosage = 0.5 ppm
1 ppm = 1 mg/L
1 mg/L = 1 mg/1000 mL
1 mg/L = 1 mg/cubic meter
Chlorine required = 0.5 mg/cubic meter
Total chlorine required = Chlorine required x city supply of water per day
Total chlorine required = 0.5 mg/cubic meter x 15000 cubic meters
Total chlorine required = 7500 mg = 7.5 g
2. Calculation of bleaching powder required
Bleaching powder is used to provide chlorine to the water
Bleaching powder contains 25% chlorine by weight
Let the weight of bleaching powder required be x kg
Chlorine provided by x kg of bleaching powder = 25% of x kg
Chlorine provided by x kg of bleaching powder = (25/100) x x kg
Chlorine provided by x kg of bleaching powder = 0.25 x kg
Chlorine provided by x kg of bleaching powder = 250 x g
As 1 g of bleaching powder contains 0.25 g of chlorine
Therefore, x kg of bleaching powder contains = (250/0.25) g of bleaching powder
x kg of bleaching powder contains = 1000 g = 1 kg of bleaching powder
3. Calculation of bleaching powder required per day
From step 2, we know that 1 kg of bleaching powder provides 250 g of chlorine
Total chlorine required per day = 7.5 g
Total bleaching powder required per day = (7.5 g / 250 g) x 1 kg
Total bleaching powder required per day = 0.03 kg = 30 kg
Therefore, the requirement of 25% bleaching powder per day would be 30 kg (option C) to treat the city supply of water with a chlorine dosage of 0.5 ppm.
City supply of water per day = 15000 cubic meters
Chlorine dosage = 0.5 ppm
To find:
Requirement of 25% bleaching powder per day
Solution:
1. Calculation of chlorine required
Chlorine dosage = 0.5 ppm
1 ppm = 1 mg/L
1 mg/L = 1 mg/1000 mL
1 mg/L = 1 mg/cubic meter
Chlorine required = 0.5 mg/cubic meter
Total chlorine required = Chlorine required x city supply of water per day
Total chlorine required = 0.5 mg/cubic meter x 15000 cubic meters
Total chlorine required = 7500 mg = 7.5 g
2. Calculation of bleaching powder required
Bleaching powder is used to provide chlorine to the water
Bleaching powder contains 25% chlorine by weight
Let the weight of bleaching powder required be x kg
Chlorine provided by x kg of bleaching powder = 25% of x kg
Chlorine provided by x kg of bleaching powder = (25/100) x x kg
Chlorine provided by x kg of bleaching powder = 0.25 x kg
Chlorine provided by x kg of bleaching powder = 250 x g
As 1 g of bleaching powder contains 0.25 g of chlorine
Therefore, x kg of bleaching powder contains = (250/0.25) g of bleaching powder
x kg of bleaching powder contains = 1000 g = 1 kg of bleaching powder
3. Calculation of bleaching powder required per day
From step 2, we know that 1 kg of bleaching powder provides 250 g of chlorine
Total chlorine required per day = 7.5 g
Total bleaching powder required per day = (7.5 g / 250 g) x 1 kg
Total bleaching powder required per day = 0.03 kg = 30 kg
Therefore, the requirement of 25% bleaching powder per day would be 30 kg (option C) to treat the city supply of water with a chlorine dosage of 0.5 ppm.
Free Test
FREE
| Start Free Test |
Community Answer
A city supply of 15000 cubic meters of water per day is treated with a...
A city must treat about 15000 m³/day of water. Flocculation particles are produced by coagulation and a column analysis indicates that an overflowrate of 20 m/day will produce satisfactory removal at a depth of 3.5m.Determine the size of a) the required rectangular settling tanks b) the required circular settling tanks

|
Explore Courses for Civil Engineering (CE) exam
|

|
Similar Civil Engineering (CE) Doubts
A city supply of 15000 cubic meters of water per day is treated with a chlorine dosage of 0.5 ppm. For this purpose, the requirement of 25% bleaching powder per day would be:a)300 kgb)75 kgc)30 kgd)750 kgCorrect answer is option 'C'. Can you explain this answer?
Question Description
A city supply of 15000 cubic meters of water per day is treated with a chlorine dosage of 0.5 ppm. For this purpose, the requirement of 25% bleaching powder per day would be:a)300 kgb)75 kgc)30 kgd)750 kgCorrect answer is option 'C'. Can you explain this answer? for Civil Engineering (CE) 2025 is part of Civil Engineering (CE) preparation. The Question and answers have been prepared according to the Civil Engineering (CE) exam syllabus. Information about A city supply of 15000 cubic meters of water per day is treated with a chlorine dosage of 0.5 ppm. For this purpose, the requirement of 25% bleaching powder per day would be:a)300 kgb)75 kgc)30 kgd)750 kgCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Civil Engineering (CE) 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for A city supply of 15000 cubic meters of water per day is treated with a chlorine dosage of 0.5 ppm. For this purpose, the requirement of 25% bleaching powder per day would be:a)300 kgb)75 kgc)30 kgd)750 kgCorrect answer is option 'C'. Can you explain this answer?.
A city supply of 15000 cubic meters of water per day is treated with a chlorine dosage of 0.5 ppm. For this purpose, the requirement of 25% bleaching powder per day would be:a)300 kgb)75 kgc)30 kgd)750 kgCorrect answer is option 'C'. Can you explain this answer? for Civil Engineering (CE) 2025 is part of Civil Engineering (CE) preparation. The Question and answers have been prepared according to the Civil Engineering (CE) exam syllabus. Information about A city supply of 15000 cubic meters of water per day is treated with a chlorine dosage of 0.5 ppm. For this purpose, the requirement of 25% bleaching powder per day would be:a)300 kgb)75 kgc)30 kgd)750 kgCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Civil Engineering (CE) 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for A city supply of 15000 cubic meters of water per day is treated with a chlorine dosage of 0.5 ppm. For this purpose, the requirement of 25% bleaching powder per day would be:a)300 kgb)75 kgc)30 kgd)750 kgCorrect answer is option 'C'. Can you explain this answer?.
Solutions for A city supply of 15000 cubic meters of water per day is treated with a chlorine dosage of 0.5 ppm. For this purpose, the requirement of 25% bleaching powder per day would be:a)300 kgb)75 kgc)30 kgd)750 kgCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Civil Engineering (CE).
Download more important topics, notes, lectures and mock test series for Civil Engineering (CE) Exam by signing up for free.
Here you can find the meaning of A city supply of 15000 cubic meters of water per day is treated with a chlorine dosage of 0.5 ppm. For this purpose, the requirement of 25% bleaching powder per day would be:a)300 kgb)75 kgc)30 kgd)750 kgCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
A city supply of 15000 cubic meters of water per day is treated with a chlorine dosage of 0.5 ppm. For this purpose, the requirement of 25% bleaching powder per day would be:a)300 kgb)75 kgc)30 kgd)750 kgCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for A city supply of 15000 cubic meters of water per day is treated with a chlorine dosage of 0.5 ppm. For this purpose, the requirement of 25% bleaching powder per day would be:a)300 kgb)75 kgc)30 kgd)750 kgCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of A city supply of 15000 cubic meters of water per day is treated with a chlorine dosage of 0.5 ppm. For this purpose, the requirement of 25% bleaching powder per day would be:a)300 kgb)75 kgc)30 kgd)750 kgCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice A city supply of 15000 cubic meters of water per day is treated with a chlorine dosage of 0.5 ppm. For this purpose, the requirement of 25% bleaching powder per day would be:a)300 kgb)75 kgc)30 kgd)750 kgCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Civil Engineering (CE) tests.

|
Explore Courses for Civil Engineering (CE) exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


























