Class 12 Exam > Class 12 Questions > An inorganic compound (A) made of two most oc...
Start Learning for Free
An inorganic compound (A) made of two most occurring elements into the earth crust, having polymeric tetra-hedral net word structure. With carbon. compound (A) produces a poisonous gas (B) which is the most stable diatomic molecule. Compounds (A) and (B) will be
- a)SiO2. CO2
- b)SiO2. CO
- c)SiC, CO
- d)SiO2. N2
Correct answer is option 'B'. Can you explain this answer?
Verified Answer
An inorganic compound (A) made of two most occurring elements into the...
SiO2 - sp3
sillica is most abundant in earthiness oxygen atom is sp3 hybridised.
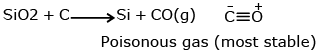
sillica is most abundant in earthiness oxygen atom is sp3 hybridised.
Most Upvoted Answer
An inorganic compound (A) made of two most occurring elements into the...
Introduction:
The question asks for an inorganic compound (A) made of two most occurring elements in the earth's crust, having a polymeric tetrahedral network structure. It also states that compound (A) produces a poisonous gas (B) which is the most stable diatomic molecule. We need to identify the correct combination of compounds (A) and (B) from the given options.
Analysis:
To solve this question, we need to consider the properties of the compounds mentioned in the options and match them with the given conditions.
Option A: SiO2 and CO2
- SiO2 is an inorganic compound made of silicon and oxygen, which are two of the most abundant elements in the earth's crust.
- CO2 is a gas produced by the combustion of carbon-containing materials, but it is not a poisonous gas.
- The combination of SiO2 and CO2 does not satisfy the given conditions.
Option B: SiO2 and CO
- SiO2 is an inorganic compound made of silicon and oxygen, which are two of the most abundant elements in the earth's crust.
- CO is a poisonous gas produced by the incomplete combustion of carbon-containing materials.
- The combination of SiO2 and CO satisfies the given conditions.
Option C: SiC and CO
- SiC is an inorganic compound made of silicon and carbon, which are two of the most abundant elements in the earth's crust.
- CO is a poisonous gas produced by the incomplete combustion of carbon-containing materials.
- The combination of SiC and CO does not satisfy the given conditions.
Option D: SiO2 and N2
- SiO2 is an inorganic compound made of silicon and oxygen, which are two of the most abundant elements in the earth's crust.
- N2 is a diatomic molecule of nitrogen gas, but it is not a poisonous gas.
- The combination of SiO2 and N2 does not satisfy the given conditions.
Conclusion:
After analyzing all the options, it is clear that the correct combination of compounds (A) and (B) is SiO2 and CO. These compounds fulfill the given conditions of being made of the two most occurring elements in the earth's crust and producing a poisonous gas, which is the most stable diatomic molecule.
The question asks for an inorganic compound (A) made of two most occurring elements in the earth's crust, having a polymeric tetrahedral network structure. It also states that compound (A) produces a poisonous gas (B) which is the most stable diatomic molecule. We need to identify the correct combination of compounds (A) and (B) from the given options.
Analysis:
To solve this question, we need to consider the properties of the compounds mentioned in the options and match them with the given conditions.
Option A: SiO2 and CO2
- SiO2 is an inorganic compound made of silicon and oxygen, which are two of the most abundant elements in the earth's crust.
- CO2 is a gas produced by the combustion of carbon-containing materials, but it is not a poisonous gas.
- The combination of SiO2 and CO2 does not satisfy the given conditions.
Option B: SiO2 and CO
- SiO2 is an inorganic compound made of silicon and oxygen, which are two of the most abundant elements in the earth's crust.
- CO is a poisonous gas produced by the incomplete combustion of carbon-containing materials.
- The combination of SiO2 and CO satisfies the given conditions.
Option C: SiC and CO
- SiC is an inorganic compound made of silicon and carbon, which are two of the most abundant elements in the earth's crust.
- CO is a poisonous gas produced by the incomplete combustion of carbon-containing materials.
- The combination of SiC and CO does not satisfy the given conditions.
Option D: SiO2 and N2
- SiO2 is an inorganic compound made of silicon and oxygen, which are two of the most abundant elements in the earth's crust.
- N2 is a diatomic molecule of nitrogen gas, but it is not a poisonous gas.
- The combination of SiO2 and N2 does not satisfy the given conditions.
Conclusion:
After analyzing all the options, it is clear that the correct combination of compounds (A) and (B) is SiO2 and CO. These compounds fulfill the given conditions of being made of the two most occurring elements in the earth's crust and producing a poisonous gas, which is the most stable diatomic molecule.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
An inorganic compound (A) made of two most occurring elements into the earth crust, having polymeric tetra-hedral net word structure. With carbon. compound (A) produces a poisonous gas (B) which is the most stable diatomic molecule. Compounds (A) and (B) will bea)SiO2. CO2b)SiO2. COc)SiC, COd)SiO2. N2Correct answer is option 'B'. Can you explain this answer?
Question Description
An inorganic compound (A) made of two most occurring elements into the earth crust, having polymeric tetra-hedral net word structure. With carbon. compound (A) produces a poisonous gas (B) which is the most stable diatomic molecule. Compounds (A) and (B) will bea)SiO2. CO2b)SiO2. COc)SiC, COd)SiO2. N2Correct answer is option 'B'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about An inorganic compound (A) made of two most occurring elements into the earth crust, having polymeric tetra-hedral net word structure. With carbon. compound (A) produces a poisonous gas (B) which is the most stable diatomic molecule. Compounds (A) and (B) will bea)SiO2. CO2b)SiO2. COc)SiC, COd)SiO2. N2Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for An inorganic compound (A) made of two most occurring elements into the earth crust, having polymeric tetra-hedral net word structure. With carbon. compound (A) produces a poisonous gas (B) which is the most stable diatomic molecule. Compounds (A) and (B) will bea)SiO2. CO2b)SiO2. COc)SiC, COd)SiO2. N2Correct answer is option 'B'. Can you explain this answer?.
An inorganic compound (A) made of two most occurring elements into the earth crust, having polymeric tetra-hedral net word structure. With carbon. compound (A) produces a poisonous gas (B) which is the most stable diatomic molecule. Compounds (A) and (B) will bea)SiO2. CO2b)SiO2. COc)SiC, COd)SiO2. N2Correct answer is option 'B'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about An inorganic compound (A) made of two most occurring elements into the earth crust, having polymeric tetra-hedral net word structure. With carbon. compound (A) produces a poisonous gas (B) which is the most stable diatomic molecule. Compounds (A) and (B) will bea)SiO2. CO2b)SiO2. COc)SiC, COd)SiO2. N2Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for An inorganic compound (A) made of two most occurring elements into the earth crust, having polymeric tetra-hedral net word structure. With carbon. compound (A) produces a poisonous gas (B) which is the most stable diatomic molecule. Compounds (A) and (B) will bea)SiO2. CO2b)SiO2. COc)SiC, COd)SiO2. N2Correct answer is option 'B'. Can you explain this answer?.
Solutions for An inorganic compound (A) made of two most occurring elements into the earth crust, having polymeric tetra-hedral net word structure. With carbon. compound (A) produces a poisonous gas (B) which is the most stable diatomic molecule. Compounds (A) and (B) will bea)SiO2. CO2b)SiO2. COc)SiC, COd)SiO2. N2Correct answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of An inorganic compound (A) made of two most occurring elements into the earth crust, having polymeric tetra-hedral net word structure. With carbon. compound (A) produces a poisonous gas (B) which is the most stable diatomic molecule. Compounds (A) and (B) will bea)SiO2. CO2b)SiO2. COc)SiC, COd)SiO2. N2Correct answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
An inorganic compound (A) made of two most occurring elements into the earth crust, having polymeric tetra-hedral net word structure. With carbon. compound (A) produces a poisonous gas (B) which is the most stable diatomic molecule. Compounds (A) and (B) will bea)SiO2. CO2b)SiO2. COc)SiC, COd)SiO2. N2Correct answer is option 'B'. Can you explain this answer?, a detailed solution for An inorganic compound (A) made of two most occurring elements into the earth crust, having polymeric tetra-hedral net word structure. With carbon. compound (A) produces a poisonous gas (B) which is the most stable diatomic molecule. Compounds (A) and (B) will bea)SiO2. CO2b)SiO2. COc)SiC, COd)SiO2. N2Correct answer is option 'B'. Can you explain this answer? has been provided alongside types of An inorganic compound (A) made of two most occurring elements into the earth crust, having polymeric tetra-hedral net word structure. With carbon. compound (A) produces a poisonous gas (B) which is the most stable diatomic molecule. Compounds (A) and (B) will bea)SiO2. CO2b)SiO2. COc)SiC, COd)SiO2. N2Correct answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice An inorganic compound (A) made of two most occurring elements into the earth crust, having polymeric tetra-hedral net word structure. With carbon. compound (A) produces a poisonous gas (B) which is the most stable diatomic molecule. Compounds (A) and (B) will bea)SiO2. CO2b)SiO2. COc)SiC, COd)SiO2. N2Correct answer is option 'B'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.



















