Class 12 Exam > Class 12 Questions > Refining of impure metal is done by electroly...
Start Learning for Free
Refining of impure metal is done by electrolysis using impure metal as anode.Select the correct statement about this refining.
- a)E°cell of the net reaction is zero
- b)metal to be refined is dissolved in the solution and deposited at the cathode.
- c)Both (a) and (b)
- d)None of the above
Correct answer is option 'C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Refining of impure metal is done by electrolysis using impure metal as...
When impure metal (M) is anode and pure metal is made cathode, then
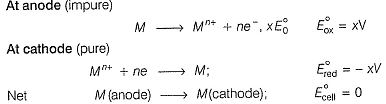
Thus, impure metal from the anode is deposited at the cathode.
Thus, impure metal from the anode is deposited at the cathode.
Most Upvoted Answer
Refining of impure metal is done by electrolysis using impure metal as...
Refining of impure metal is done by electrolysis using the impure metal as the anode. This process is known as electrorefining or electrolytic refining. It is commonly used to purify metals such as copper, zinc, and nickel.
Ecell of the net reaction is zero:
- The Ecell (cell potential) of the net reaction during the electrorefining process is not zero. In fact, it is a positive value. This means that the reaction is spontaneous and can proceed without the need for an external energy source. The positive Ecell indicates that the impure metal at the anode is oxidized and ions are being reduced at the cathode.
Metal to be refined is dissolved in the solution and deposited at the cathode:
- The metal to be refined is indeed dissolved in the electrolyte solution during the electrorefining process. When an electric current is passed through the electrolyte, metal cations from the impure metal at the anode are transferred into the electrolyte as metal ions. These metal ions then migrate towards the cathode, where they are reduced and deposited as pure metal. This deposition of pure metal at the cathode helps in the purification process.
Both (a) and (b):
- The correct statement about the refining of impure metal by electrolysis is that both statement (a) and (b) are correct. The Ecell of the net reaction is not zero, but a positive value indicating the spontaneity of the process. Additionally, the metal to be refined is dissolved in the electrolyte solution and deposited at the cathode, leading to the purification of the metal.
None of the above:
- This option is incorrect as both statement (a) and (b) are correct. The Ecell is not zero, and the metal is dissolved in the solution and deposited at the cathode during the electrorefining process.
In conclusion, the correct statement about the refining of impure metal by electrolysis is that both statement (a) and (b) are correct.
Ecell of the net reaction is zero:
- The Ecell (cell potential) of the net reaction during the electrorefining process is not zero. In fact, it is a positive value. This means that the reaction is spontaneous and can proceed without the need for an external energy source. The positive Ecell indicates that the impure metal at the anode is oxidized and ions are being reduced at the cathode.
Metal to be refined is dissolved in the solution and deposited at the cathode:
- The metal to be refined is indeed dissolved in the electrolyte solution during the electrorefining process. When an electric current is passed through the electrolyte, metal cations from the impure metal at the anode are transferred into the electrolyte as metal ions. These metal ions then migrate towards the cathode, where they are reduced and deposited as pure metal. This deposition of pure metal at the cathode helps in the purification process.
Both (a) and (b):
- The correct statement about the refining of impure metal by electrolysis is that both statement (a) and (b) are correct. The Ecell of the net reaction is not zero, but a positive value indicating the spontaneity of the process. Additionally, the metal to be refined is dissolved in the electrolyte solution and deposited at the cathode, leading to the purification of the metal.
None of the above:
- This option is incorrect as both statement (a) and (b) are correct. The Ecell is not zero, and the metal is dissolved in the solution and deposited at the cathode during the electrorefining process.
In conclusion, the correct statement about the refining of impure metal by electrolysis is that both statement (a) and (b) are correct.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
Refining of impure metal is done by electrolysis using impure metal as anode.Select the correct statement about this refining.a)E°cell of the net reaction is zerob)metal to be refined is dissolved in the solution and deposited at the cathode.c)Both (a) and (b)d)None of the aboveCorrect answer is option 'C'. Can you explain this answer?
Question Description
Refining of impure metal is done by electrolysis using impure metal as anode.Select the correct statement about this refining.a)E°cell of the net reaction is zerob)metal to be refined is dissolved in the solution and deposited at the cathode.c)Both (a) and (b)d)None of the aboveCorrect answer is option 'C'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Refining of impure metal is done by electrolysis using impure metal as anode.Select the correct statement about this refining.a)E°cell of the net reaction is zerob)metal to be refined is dissolved in the solution and deposited at the cathode.c)Both (a) and (b)d)None of the aboveCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Refining of impure metal is done by electrolysis using impure metal as anode.Select the correct statement about this refining.a)E°cell of the net reaction is zerob)metal to be refined is dissolved in the solution and deposited at the cathode.c)Both (a) and (b)d)None of the aboveCorrect answer is option 'C'. Can you explain this answer?.
Refining of impure metal is done by electrolysis using impure metal as anode.Select the correct statement about this refining.a)E°cell of the net reaction is zerob)metal to be refined is dissolved in the solution and deposited at the cathode.c)Both (a) and (b)d)None of the aboveCorrect answer is option 'C'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Refining of impure metal is done by electrolysis using impure metal as anode.Select the correct statement about this refining.a)E°cell of the net reaction is zerob)metal to be refined is dissolved in the solution and deposited at the cathode.c)Both (a) and (b)d)None of the aboveCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Refining of impure metal is done by electrolysis using impure metal as anode.Select the correct statement about this refining.a)E°cell of the net reaction is zerob)metal to be refined is dissolved in the solution and deposited at the cathode.c)Both (a) and (b)d)None of the aboveCorrect answer is option 'C'. Can you explain this answer?.
Solutions for Refining of impure metal is done by electrolysis using impure metal as anode.Select the correct statement about this refining.a)E°cell of the net reaction is zerob)metal to be refined is dissolved in the solution and deposited at the cathode.c)Both (a) and (b)d)None of the aboveCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Refining of impure metal is done by electrolysis using impure metal as anode.Select the correct statement about this refining.a)E°cell of the net reaction is zerob)metal to be refined is dissolved in the solution and deposited at the cathode.c)Both (a) and (b)d)None of the aboveCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Refining of impure metal is done by electrolysis using impure metal as anode.Select the correct statement about this refining.a)E°cell of the net reaction is zerob)metal to be refined is dissolved in the solution and deposited at the cathode.c)Both (a) and (b)d)None of the aboveCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for Refining of impure metal is done by electrolysis using impure metal as anode.Select the correct statement about this refining.a)E°cell of the net reaction is zerob)metal to be refined is dissolved in the solution and deposited at the cathode.c)Both (a) and (b)d)None of the aboveCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of Refining of impure metal is done by electrolysis using impure metal as anode.Select the correct statement about this refining.a)E°cell of the net reaction is zerob)metal to be refined is dissolved in the solution and deposited at the cathode.c)Both (a) and (b)d)None of the aboveCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Refining of impure metal is done by electrolysis using impure metal as anode.Select the correct statement about this refining.a)E°cell of the net reaction is zerob)metal to be refined is dissolved in the solution and deposited at the cathode.c)Both (a) and (b)d)None of the aboveCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















