Class 12 Exam > Class 12 Questions > In the following batteries, alkaline electrol...
Start Learning for Free
In the following batteries, alkaline electrolytes are used
I. Mercury
II. Nickel-cadmium
III. Modified Leclanche cell Cell potential is found to be independent of [OH-] in
II. Nickel-cadmium
III. Modified Leclanche cell Cell potential is found to be independent of [OH-] in
- a)I , II , III
- b)I ,II
- c)II , III
- d)Only III
Correct answer is option 'A'. Can you explain this answer?
Verified Answer
In the following batteries, alkaline electrolytes are usedI. MercuryII...
I. Mercury battery has anode made of zinc and cathode is a steel vessel in contact with HgO in alkaline medium of KOH and Zn(OH)2.
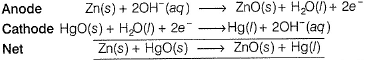
Net reaction does not involve OH- , hence emf is independent of [OH-].
II. In nickel, cadmium battery, net reaction is
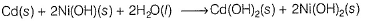
Since, (OH-) species are in solid state, hence emf is independent of (OH-).
III. Alkaline dry cell is a modified version of the Leclanche cell. Net reaction is
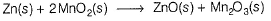
Thus, emf is independent of (OH-).
Net reaction does not involve OH- , hence emf is independent of [OH-].
II. In nickel, cadmium battery, net reaction is
Since, (OH-) species are in solid state, hence emf is independent of (OH-).
III. Alkaline dry cell is a modified version of the Leclanche cell. Net reaction is
Thus, emf is independent of (OH-).
Most Upvoted Answer
In the following batteries, alkaline electrolytes are usedI. MercuryII...
Mercury Battery:
- Mercury batteries, also known as mercuric oxide batteries, use a combination of mercuric oxide and zinc as the electrode materials.
- The electrolyte in mercury batteries is typically an alkaline electrolyte, which is a solution of potassium hydroxide (KOH) or sodium hydroxide (NaOH).
- The alkaline electrolyte facilitates the flow of ions between the electrodes, allowing the battery to generate an electric current.
- The cell potential of a mercury battery is not dependent on the concentration of hydroxide ions ([OH-]) in the electrolyte. Therefore, the cell potential remains constant regardless of the [OH-] concentration.
Nickel-Cadmium Battery:
- Nickel-cadmium batteries, also known as NiCd batteries, use nickel oxide hydroxide and metallic cadmium as the electrode materials.
- The electrolyte in NiCd batteries is typically an alkaline electrolyte, which is usually a solution of potassium hydroxide (KOH).
- Similar to mercury batteries, the alkaline electrolyte in NiCd batteries facilitates the flow of ions between the electrodes, allowing the battery to generate an electric current.
- The cell potential of a NiCd battery is also not dependent on the concentration of hydroxide ions ([OH-]) in the electrolyte. Therefore, the cell potential remains constant regardless of the [OH-] concentration.
Modified Leclanche Cell:
- The Leclanche cell is a type of primary battery that uses a zinc anode and a carbon cathode.
- In a modified Leclanche cell, the electrolyte is typically a paste made of ammonium chloride (NH4Cl) and zinc chloride (ZnCl2) dissolved in water.
- The cell potential of a modified Leclanche cell is not dependent on the concentration of hydroxide ions ([OH-]) in the electrolyte. Therefore, the cell potential remains constant regardless of the [OH-] concentration.
Overall Explanation:
- Alkaline electrolytes, such as solutions of potassium hydroxide (KOH) or sodium hydroxide (NaOH), are commonly used in different types of batteries to facilitate ion flow and enable the generation of electric current.
- In the case of mercury batteries, nickel-cadmium batteries, and modified Leclanche cells, alkaline electrolytes are used.
- The cell potential of these batteries is not affected by the concentration of hydroxide ions ([OH-]) in the electrolyte.
- Therefore, the cell potential remains constant regardless of the [OH-] concentration.
- As a result, the correct answer to the given question is option 'A' - I, II, III, meaning that all three types of batteries mentioned (mercury battery, nickel-cadmium battery, and modified Leclanche cell) use alkaline electrolytes and have a cell potential that is independent of [OH-] concentration.
- Mercury batteries, also known as mercuric oxide batteries, use a combination of mercuric oxide and zinc as the electrode materials.
- The electrolyte in mercury batteries is typically an alkaline electrolyte, which is a solution of potassium hydroxide (KOH) or sodium hydroxide (NaOH).
- The alkaline electrolyte facilitates the flow of ions between the electrodes, allowing the battery to generate an electric current.
- The cell potential of a mercury battery is not dependent on the concentration of hydroxide ions ([OH-]) in the electrolyte. Therefore, the cell potential remains constant regardless of the [OH-] concentration.
Nickel-Cadmium Battery:
- Nickel-cadmium batteries, also known as NiCd batteries, use nickel oxide hydroxide and metallic cadmium as the electrode materials.
- The electrolyte in NiCd batteries is typically an alkaline electrolyte, which is usually a solution of potassium hydroxide (KOH).
- Similar to mercury batteries, the alkaline electrolyte in NiCd batteries facilitates the flow of ions between the electrodes, allowing the battery to generate an electric current.
- The cell potential of a NiCd battery is also not dependent on the concentration of hydroxide ions ([OH-]) in the electrolyte. Therefore, the cell potential remains constant regardless of the [OH-] concentration.
Modified Leclanche Cell:
- The Leclanche cell is a type of primary battery that uses a zinc anode and a carbon cathode.
- In a modified Leclanche cell, the electrolyte is typically a paste made of ammonium chloride (NH4Cl) and zinc chloride (ZnCl2) dissolved in water.
- The cell potential of a modified Leclanche cell is not dependent on the concentration of hydroxide ions ([OH-]) in the electrolyte. Therefore, the cell potential remains constant regardless of the [OH-] concentration.
Overall Explanation:
- Alkaline electrolytes, such as solutions of potassium hydroxide (KOH) or sodium hydroxide (NaOH), are commonly used in different types of batteries to facilitate ion flow and enable the generation of electric current.
- In the case of mercury batteries, nickel-cadmium batteries, and modified Leclanche cells, alkaline electrolytes are used.
- The cell potential of these batteries is not affected by the concentration of hydroxide ions ([OH-]) in the electrolyte.
- Therefore, the cell potential remains constant regardless of the [OH-] concentration.
- As a result, the correct answer to the given question is option 'A' - I, II, III, meaning that all three types of batteries mentioned (mercury battery, nickel-cadmium battery, and modified Leclanche cell) use alkaline electrolytes and have a cell potential that is independent of [OH-] concentration.

|
Explore Courses for Class 12 exam
|

|
Question Description
In the following batteries, alkaline electrolytes are usedI. MercuryII. Nickel-cadmiumIII. Modified Leclanche cell Cell potential is found to be independent of [OH-] ina)I , II , IIIb)I ,IIc)II , IIId)Only IIICorrect answer is option 'A'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about In the following batteries, alkaline electrolytes are usedI. MercuryII. Nickel-cadmiumIII. Modified Leclanche cell Cell potential is found to be independent of [OH-] ina)I , II , IIIb)I ,IIc)II , IIId)Only IIICorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In the following batteries, alkaline electrolytes are usedI. MercuryII. Nickel-cadmiumIII. Modified Leclanche cell Cell potential is found to be independent of [OH-] ina)I , II , IIIb)I ,IIc)II , IIId)Only IIICorrect answer is option 'A'. Can you explain this answer?.
In the following batteries, alkaline electrolytes are usedI. MercuryII. Nickel-cadmiumIII. Modified Leclanche cell Cell potential is found to be independent of [OH-] ina)I , II , IIIb)I ,IIc)II , IIId)Only IIICorrect answer is option 'A'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about In the following batteries, alkaline electrolytes are usedI. MercuryII. Nickel-cadmiumIII. Modified Leclanche cell Cell potential is found to be independent of [OH-] ina)I , II , IIIb)I ,IIc)II , IIId)Only IIICorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In the following batteries, alkaline electrolytes are usedI. MercuryII. Nickel-cadmiumIII. Modified Leclanche cell Cell potential is found to be independent of [OH-] ina)I , II , IIIb)I ,IIc)II , IIId)Only IIICorrect answer is option 'A'. Can you explain this answer?.
Solutions for In the following batteries, alkaline electrolytes are usedI. MercuryII. Nickel-cadmiumIII. Modified Leclanche cell Cell potential is found to be independent of [OH-] ina)I , II , IIIb)I ,IIc)II , IIId)Only IIICorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of In the following batteries, alkaline electrolytes are usedI. MercuryII. Nickel-cadmiumIII. Modified Leclanche cell Cell potential is found to be independent of [OH-] ina)I , II , IIIb)I ,IIc)II , IIId)Only IIICorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
In the following batteries, alkaline electrolytes are usedI. MercuryII. Nickel-cadmiumIII. Modified Leclanche cell Cell potential is found to be independent of [OH-] ina)I , II , IIIb)I ,IIc)II , IIId)Only IIICorrect answer is option 'A'. Can you explain this answer?, a detailed solution for In the following batteries, alkaline electrolytes are usedI. MercuryII. Nickel-cadmiumIII. Modified Leclanche cell Cell potential is found to be independent of [OH-] ina)I , II , IIIb)I ,IIc)II , IIId)Only IIICorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of In the following batteries, alkaline electrolytes are usedI. MercuryII. Nickel-cadmiumIII. Modified Leclanche cell Cell potential is found to be independent of [OH-] ina)I , II , IIIb)I ,IIc)II , IIId)Only IIICorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice In the following batteries, alkaline electrolytes are usedI. MercuryII. Nickel-cadmiumIII. Modified Leclanche cell Cell potential is found to be independent of [OH-] ina)I , II , IIIb)I ,IIc)II , IIId)Only IIICorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















