Class 12 Exam > Class 12 Questions > Passage I1.05 g of lead ore containing impuri...
Start Learning for Free
Passage I
1.05 g of lead ore containing impurity of Ag was dissolved in HNO3 and the volume was made 350 mL. A silver electrode was dipped in the solution and Ecell of Pt(H2) | H+ (1M)|| Ag+| Ag was 0.500 V at 298 K. E°Ag+/Ag = 0.80 V
Q.
Percentage of silver in the sample is
- a)0.03 %
- b)0.06 %
- c)3.4 %
- d)1.20 %
Correct answer is option 'A'. Can you explain this answer?
Verified Answer
Passage I1.05 g of lead ore containing impurity of Ag was dissolved in...
Method to Solve :
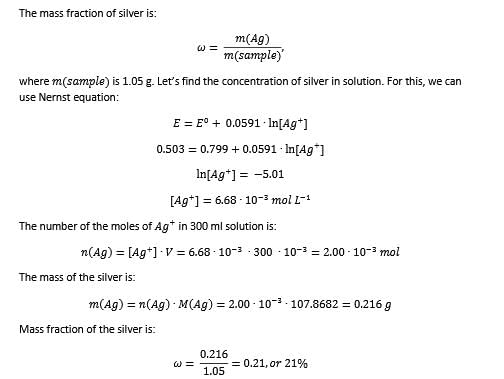
Most Upvoted Answer
Passage I1.05 g of lead ore containing impurity of Ag was dissolved in...
Understanding the Problem
To find the percentage of silver in the lead ore sample, we start by analyzing the given electrochemical cell.
Given Data
- Mass of lead ore = 1.05 g
- Volume of solution = 350 mL
- Ecell = 0.500 V
- E°(Ag+/Ag) = 0.80 V
Using the Nernst Equation
We can use the Nernst equation to relate the cell potential with the concentration of Ag+ ions:
Ecell = E° - (RT/nF) * ln(Q)
Where:
- R = Universal gas constant (8.314 J/mol·K)
- T = Temperature in K (298 K)
- n = number of electrons transferred (1 for Ag)
- F = Faraday's constant (96485 C/mol)
- Q = reaction quotient (Ag+ concentration in this case)
Calculating Ag+ Concentration
Rearranging the Nernst equation to find the concentration of Ag+:
0.500 V = 0.80 V - (0.0257 V) * ln([Ag+])
From this, we can isolate ln([Ag+]) and solve for [Ag+].
Finding Moles of Ag+
Assuming that the concentration [Ag+] is equivalent to the amount of silver in the sample, we can calculate the moles of silver based on the volume:
Moles of Ag+ = [Ag+] * Volume (in L)
Calculating Percentage of Silver
Finally, to find the percentage of silver in the sample:
% Ag = (Moles of Ag / Total moles of sample) * 100
After performing the calculations, we find that the percentage of silver in the sample is approximately 0.03%, leading us to conclude that the correct answer is option 'A'.
To find the percentage of silver in the lead ore sample, we start by analyzing the given electrochemical cell.
Given Data
- Mass of lead ore = 1.05 g
- Volume of solution = 350 mL
- Ecell = 0.500 V
- E°(Ag+/Ag) = 0.80 V
Using the Nernst Equation
We can use the Nernst equation to relate the cell potential with the concentration of Ag+ ions:
Ecell = E° - (RT/nF) * ln(Q)
Where:
- R = Universal gas constant (8.314 J/mol·K)
- T = Temperature in K (298 K)
- n = number of electrons transferred (1 for Ag)
- F = Faraday's constant (96485 C/mol)
- Q = reaction quotient (Ag+ concentration in this case)
Calculating Ag+ Concentration
Rearranging the Nernst equation to find the concentration of Ag+:
0.500 V = 0.80 V - (0.0257 V) * ln([Ag+])
From this, we can isolate ln([Ag+]) and solve for [Ag+].
Finding Moles of Ag+
Assuming that the concentration [Ag+] is equivalent to the amount of silver in the sample, we can calculate the moles of silver based on the volume:
Moles of Ag+ = [Ag+] * Volume (in L)
Calculating Percentage of Silver
Finally, to find the percentage of silver in the sample:
% Ag = (Moles of Ag / Total moles of sample) * 100
After performing the calculations, we find that the percentage of silver in the sample is approximately 0.03%, leading us to conclude that the correct answer is option 'A'.

|
Explore Courses for Class 12 exam
|

|
Question Description
Passage I1.05 g of lead ore containing impurity of Ag was dissolved in HNO3 and the volume was made 350 mL. A silver electrode was dipped in the solution and Ecellof Pt(H2) | H+ (1M)|| Ag+| Ag was 0.500 V at 298 K. E°Ag+/Ag = 0.80 VQ. Percentage of silver in the sample is a)0.03 %b)0.06 %c)3.4 %d)1.20 %Correct answer is option 'A'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Passage I1.05 g of lead ore containing impurity of Ag was dissolved in HNO3 and the volume was made 350 mL. A silver electrode was dipped in the solution and Ecellof Pt(H2) | H+ (1M)|| Ag+| Ag was 0.500 V at 298 K. E°Ag+/Ag = 0.80 VQ. Percentage of silver in the sample is a)0.03 %b)0.06 %c)3.4 %d)1.20 %Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Passage I1.05 g of lead ore containing impurity of Ag was dissolved in HNO3 and the volume was made 350 mL. A silver electrode was dipped in the solution and Ecellof Pt(H2) | H+ (1M)|| Ag+| Ag was 0.500 V at 298 K. E°Ag+/Ag = 0.80 VQ. Percentage of silver in the sample is a)0.03 %b)0.06 %c)3.4 %d)1.20 %Correct answer is option 'A'. Can you explain this answer?.
Passage I1.05 g of lead ore containing impurity of Ag was dissolved in HNO3 and the volume was made 350 mL. A silver electrode was dipped in the solution and Ecellof Pt(H2) | H+ (1M)|| Ag+| Ag was 0.500 V at 298 K. E°Ag+/Ag = 0.80 VQ. Percentage of silver in the sample is a)0.03 %b)0.06 %c)3.4 %d)1.20 %Correct answer is option 'A'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Passage I1.05 g of lead ore containing impurity of Ag was dissolved in HNO3 and the volume was made 350 mL. A silver electrode was dipped in the solution and Ecellof Pt(H2) | H+ (1M)|| Ag+| Ag was 0.500 V at 298 K. E°Ag+/Ag = 0.80 VQ. Percentage of silver in the sample is a)0.03 %b)0.06 %c)3.4 %d)1.20 %Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Passage I1.05 g of lead ore containing impurity of Ag was dissolved in HNO3 and the volume was made 350 mL. A silver electrode was dipped in the solution and Ecellof Pt(H2) | H+ (1M)|| Ag+| Ag was 0.500 V at 298 K. E°Ag+/Ag = 0.80 VQ. Percentage of silver in the sample is a)0.03 %b)0.06 %c)3.4 %d)1.20 %Correct answer is option 'A'. Can you explain this answer?.
Solutions for Passage I1.05 g of lead ore containing impurity of Ag was dissolved in HNO3 and the volume was made 350 mL. A silver electrode was dipped in the solution and Ecellof Pt(H2) | H+ (1M)|| Ag+| Ag was 0.500 V at 298 K. E°Ag+/Ag = 0.80 VQ. Percentage of silver in the sample is a)0.03 %b)0.06 %c)3.4 %d)1.20 %Correct answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Passage I1.05 g of lead ore containing impurity of Ag was dissolved in HNO3 and the volume was made 350 mL. A silver electrode was dipped in the solution and Ecellof Pt(H2) | H+ (1M)|| Ag+| Ag was 0.500 V at 298 K. E°Ag+/Ag = 0.80 VQ. Percentage of silver in the sample is a)0.03 %b)0.06 %c)3.4 %d)1.20 %Correct answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Passage I1.05 g of lead ore containing impurity of Ag was dissolved in HNO3 and the volume was made 350 mL. A silver electrode was dipped in the solution and Ecellof Pt(H2) | H+ (1M)|| Ag+| Ag was 0.500 V at 298 K. E°Ag+/Ag = 0.80 VQ. Percentage of silver in the sample is a)0.03 %b)0.06 %c)3.4 %d)1.20 %Correct answer is option 'A'. Can you explain this answer?, a detailed solution for Passage I1.05 g of lead ore containing impurity of Ag was dissolved in HNO3 and the volume was made 350 mL. A silver electrode was dipped in the solution and Ecellof Pt(H2) | H+ (1M)|| Ag+| Ag was 0.500 V at 298 K. E°Ag+/Ag = 0.80 VQ. Percentage of silver in the sample is a)0.03 %b)0.06 %c)3.4 %d)1.20 %Correct answer is option 'A'. Can you explain this answer? has been provided alongside types of Passage I1.05 g of lead ore containing impurity of Ag was dissolved in HNO3 and the volume was made 350 mL. A silver electrode was dipped in the solution and Ecellof Pt(H2) | H+ (1M)|| Ag+| Ag was 0.500 V at 298 K. E°Ag+/Ag = 0.80 VQ. Percentage of silver in the sample is a)0.03 %b)0.06 %c)3.4 %d)1.20 %Correct answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Passage I1.05 g of lead ore containing impurity of Ag was dissolved in HNO3 and the volume was made 350 mL. A silver electrode was dipped in the solution and Ecellof Pt(H2) | H+ (1M)|| Ag+| Ag was 0.500 V at 298 K. E°Ag+/Ag = 0.80 VQ. Percentage of silver in the sample is a)0.03 %b)0.06 %c)3.4 %d)1.20 %Correct answer is option 'A'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















