Class 12 Exam > Class 12 Questions > How many different isomers of amine exist for...
Start Learning for Free
How many different isomers of amine exist for C5H13N that on treatment with tosyl chloride form precipitate which does not dissolve in KOH solution?
Correct answer is '7'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
How many different isomers of amine exist for C5H13N that on treatment...
Secondary amines form sulphonamides that do not dissolve in KOH solution.
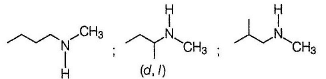
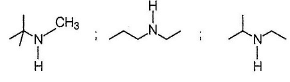
Most Upvoted Answer
How many different isomers of amine exist for C5H13N that on treatment...
There are several isomers of amine with the molecular formula C5H13N that form a precipitate when treated with tosyl chloride and do not dissolve in a KOH solution. The correct answer is '7'. Let's break down the reasoning behind this answer.
1. **Molecular Formula**: The given molecular formula is C5H13N, which means there are 5 carbon atoms, 13 hydrogen atoms, and 1 nitrogen atom in the molecule.
2. **Functional Group**: The functional group present in the given molecular formula is an amine group (-NH2).
3. **Reaction with Tosyl Chloride**: When an amine reacts with tosyl chloride (TsCl), it forms a tosylate ester. This reaction is known as the tosylation reaction. The tosylate ester is insoluble in water and forms a precipitate.
4. **Insolubility in KOH Solution**: A compound is insoluble in KOH solution if it does not react with KOH or if the resulting product is insoluble in water. In this case, the tosylate ester formed from the reaction of the amine with tosyl chloride is insoluble in KOH solution.
Based on these criteria, we can determine the number of different isomers of amine that satisfy these conditions.
- **Cyclic Amines**: Cyclic amines with 5 carbon atoms and one nitrogen atom are excluded from this analysis because they do not have enough hydrogen atoms to accommodate the amine group and the tosyl group simultaneously.
- **Aliphatic Amines**: Aliphatic amines with 5 carbon atoms can have different structures. The possible isomers can be categorized based on the position of the amine group within the carbon chain.
a. **Primary Amines**: Primary amines have the amine group attached to a terminal carbon atom.
b. **Secondary Amines**: Secondary amines have the amine group attached to a carbon atom that is bonded to two other carbon atoms.
c. **Tertiary Amines**: Tertiary amines have the amine group attached to a carbon atom that is bonded to three other carbon atoms.
d. **Quaternary Ammonium Salts**: Quaternary ammonium salts have a positively charged nitrogen atom and four alkyl or aryl groups attached to it. These compounds do not form a precipitate with tosyl chloride and are excluded from the analysis.
After considering the possible isomers and their structures, it is found that there are a total of 7 different isomers of amine with the molecular formula C5H13N that satisfy the given conditions.
1. **Molecular Formula**: The given molecular formula is C5H13N, which means there are 5 carbon atoms, 13 hydrogen atoms, and 1 nitrogen atom in the molecule.
2. **Functional Group**: The functional group present in the given molecular formula is an amine group (-NH2).
3. **Reaction with Tosyl Chloride**: When an amine reacts with tosyl chloride (TsCl), it forms a tosylate ester. This reaction is known as the tosylation reaction. The tosylate ester is insoluble in water and forms a precipitate.
4. **Insolubility in KOH Solution**: A compound is insoluble in KOH solution if it does not react with KOH or if the resulting product is insoluble in water. In this case, the tosylate ester formed from the reaction of the amine with tosyl chloride is insoluble in KOH solution.
Based on these criteria, we can determine the number of different isomers of amine that satisfy these conditions.
- **Cyclic Amines**: Cyclic amines with 5 carbon atoms and one nitrogen atom are excluded from this analysis because they do not have enough hydrogen atoms to accommodate the amine group and the tosyl group simultaneously.
- **Aliphatic Amines**: Aliphatic amines with 5 carbon atoms can have different structures. The possible isomers can be categorized based on the position of the amine group within the carbon chain.
a. **Primary Amines**: Primary amines have the amine group attached to a terminal carbon atom.
b. **Secondary Amines**: Secondary amines have the amine group attached to a carbon atom that is bonded to two other carbon atoms.
c. **Tertiary Amines**: Tertiary amines have the amine group attached to a carbon atom that is bonded to three other carbon atoms.
d. **Quaternary Ammonium Salts**: Quaternary ammonium salts have a positively charged nitrogen atom and four alkyl or aryl groups attached to it. These compounds do not form a precipitate with tosyl chloride and are excluded from the analysis.
After considering the possible isomers and their structures, it is found that there are a total of 7 different isomers of amine with the molecular formula C5H13N that satisfy the given conditions.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
How many different isomers of amine exist for C5H13N that on treatment with tosyl chloride form precipitate which does not dissolve in KOH solution?Correct answer is '7'. Can you explain this answer?
Question Description
How many different isomers of amine exist for C5H13N that on treatment with tosyl chloride form precipitate which does not dissolve in KOH solution?Correct answer is '7'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about How many different isomers of amine exist for C5H13N that on treatment with tosyl chloride form precipitate which does not dissolve in KOH solution?Correct answer is '7'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for How many different isomers of amine exist for C5H13N that on treatment with tosyl chloride form precipitate which does not dissolve in KOH solution?Correct answer is '7'. Can you explain this answer?.
How many different isomers of amine exist for C5H13N that on treatment with tosyl chloride form precipitate which does not dissolve in KOH solution?Correct answer is '7'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about How many different isomers of amine exist for C5H13N that on treatment with tosyl chloride form precipitate which does not dissolve in KOH solution?Correct answer is '7'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for How many different isomers of amine exist for C5H13N that on treatment with tosyl chloride form precipitate which does not dissolve in KOH solution?Correct answer is '7'. Can you explain this answer?.
Solutions for How many different isomers of amine exist for C5H13N that on treatment with tosyl chloride form precipitate which does not dissolve in KOH solution?Correct answer is '7'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of How many different isomers of amine exist for C5H13N that on treatment with tosyl chloride form precipitate which does not dissolve in KOH solution?Correct answer is '7'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
How many different isomers of amine exist for C5H13N that on treatment with tosyl chloride form precipitate which does not dissolve in KOH solution?Correct answer is '7'. Can you explain this answer?, a detailed solution for How many different isomers of amine exist for C5H13N that on treatment with tosyl chloride form precipitate which does not dissolve in KOH solution?Correct answer is '7'. Can you explain this answer? has been provided alongside types of How many different isomers of amine exist for C5H13N that on treatment with tosyl chloride form precipitate which does not dissolve in KOH solution?Correct answer is '7'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice How many different isomers of amine exist for C5H13N that on treatment with tosyl chloride form precipitate which does not dissolve in KOH solution?Correct answer is '7'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















