JEE Exam > JEE Questions > PASSAGE - 2There are some deposits of nitrate...
Start Learning for Free
PASSAGE - 2
There are some deposits of nitrates and phosphates in earth’s crust. Nitrates are more soluble in water. Nitrates are difficult to reduce under the laboratory conditions but microbes do it easily.
Ammonia forms large number of complexes with transition metal ions. Hybridization easily explains the ease of sigma donation capability of NH3 and PH3. Phosphine is a flammable gas and is prepared from white phosphorous.
Ammonia forms large number of complexes with transition metal ions. Hybridization easily explains the ease of sigma donation capability of NH3 and PH3. Phosphine is a flammable gas and is prepared from white phosphorous.
Q. White phosphorus on reaction with NaOH gives PH3 as one of the products. This is a
- a)dimerization reaction
- b)disporportionation reaction
- c)condensation reaction
- d)precipitation reaction
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
PASSAGE - 2There are some deposits of nitrates and phosphates in earth...
The reaction between NaOH and white phosphorus (P4) can be represented as follows:
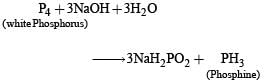
NOTE : In this reaction Phosphorus is oxidised as well as reduced so it is a disproportionation reaction.
∴ The correct answer is (b).

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
PASSAGE - 2There are some deposits of nitrates and phosphates in earth’s crust. Nitrates are more soluble in water. Nitrates are difficult to reduce under the laboratory conditions but microbes do it easily.Ammonia forms large number of complexes with transition metal ions. Hybridization easily explains the ease of sigma donation capability of NH3 and PH3. Phosphine is a flammable gas and is prepared from white phosphorous.Q.White phosphorus on reaction with NaOH gives PH3 as one of the products. This is aa)dimerization reactionb)disporportionation reactionc)condensation reactiond)precipitation reactionCorrect answer is option 'B'. Can you explain this answer?
Question Description
PASSAGE - 2There are some deposits of nitrates and phosphates in earth’s crust. Nitrates are more soluble in water. Nitrates are difficult to reduce under the laboratory conditions but microbes do it easily.Ammonia forms large number of complexes with transition metal ions. Hybridization easily explains the ease of sigma donation capability of NH3 and PH3. Phosphine is a flammable gas and is prepared from white phosphorous.Q.White phosphorus on reaction with NaOH gives PH3 as one of the products. This is aa)dimerization reactionb)disporportionation reactionc)condensation reactiond)precipitation reactionCorrect answer is option 'B'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about PASSAGE - 2There are some deposits of nitrates and phosphates in earth’s crust. Nitrates are more soluble in water. Nitrates are difficult to reduce under the laboratory conditions but microbes do it easily.Ammonia forms large number of complexes with transition metal ions. Hybridization easily explains the ease of sigma donation capability of NH3 and PH3. Phosphine is a flammable gas and is prepared from white phosphorous.Q.White phosphorus on reaction with NaOH gives PH3 as one of the products. This is aa)dimerization reactionb)disporportionation reactionc)condensation reactiond)precipitation reactionCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for PASSAGE - 2There are some deposits of nitrates and phosphates in earth’s crust. Nitrates are more soluble in water. Nitrates are difficult to reduce under the laboratory conditions but microbes do it easily.Ammonia forms large number of complexes with transition metal ions. Hybridization easily explains the ease of sigma donation capability of NH3 and PH3. Phosphine is a flammable gas and is prepared from white phosphorous.Q.White phosphorus on reaction with NaOH gives PH3 as one of the products. This is aa)dimerization reactionb)disporportionation reactionc)condensation reactiond)precipitation reactionCorrect answer is option 'B'. Can you explain this answer?.
PASSAGE - 2There are some deposits of nitrates and phosphates in earth’s crust. Nitrates are more soluble in water. Nitrates are difficult to reduce under the laboratory conditions but microbes do it easily.Ammonia forms large number of complexes with transition metal ions. Hybridization easily explains the ease of sigma donation capability of NH3 and PH3. Phosphine is a flammable gas and is prepared from white phosphorous.Q.White phosphorus on reaction with NaOH gives PH3 as one of the products. This is aa)dimerization reactionb)disporportionation reactionc)condensation reactiond)precipitation reactionCorrect answer is option 'B'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about PASSAGE - 2There are some deposits of nitrates and phosphates in earth’s crust. Nitrates are more soluble in water. Nitrates are difficult to reduce under the laboratory conditions but microbes do it easily.Ammonia forms large number of complexes with transition metal ions. Hybridization easily explains the ease of sigma donation capability of NH3 and PH3. Phosphine is a flammable gas and is prepared from white phosphorous.Q.White phosphorus on reaction with NaOH gives PH3 as one of the products. This is aa)dimerization reactionb)disporportionation reactionc)condensation reactiond)precipitation reactionCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for PASSAGE - 2There are some deposits of nitrates and phosphates in earth’s crust. Nitrates are more soluble in water. Nitrates are difficult to reduce under the laboratory conditions but microbes do it easily.Ammonia forms large number of complexes with transition metal ions. Hybridization easily explains the ease of sigma donation capability of NH3 and PH3. Phosphine is a flammable gas and is prepared from white phosphorous.Q.White phosphorus on reaction with NaOH gives PH3 as one of the products. This is aa)dimerization reactionb)disporportionation reactionc)condensation reactiond)precipitation reactionCorrect answer is option 'B'. Can you explain this answer?.
Solutions for PASSAGE - 2There are some deposits of nitrates and phosphates in earth’s crust. Nitrates are more soluble in water. Nitrates are difficult to reduce under the laboratory conditions but microbes do it easily.Ammonia forms large number of complexes with transition metal ions. Hybridization easily explains the ease of sigma donation capability of NH3 and PH3. Phosphine is a flammable gas and is prepared from white phosphorous.Q.White phosphorus on reaction with NaOH gives PH3 as one of the products. This is aa)dimerization reactionb)disporportionation reactionc)condensation reactiond)precipitation reactionCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of PASSAGE - 2There are some deposits of nitrates and phosphates in earth’s crust. Nitrates are more soluble in water. Nitrates are difficult to reduce under the laboratory conditions but microbes do it easily.Ammonia forms large number of complexes with transition metal ions. Hybridization easily explains the ease of sigma donation capability of NH3 and PH3. Phosphine is a flammable gas and is prepared from white phosphorous.Q.White phosphorus on reaction with NaOH gives PH3 as one of the products. This is aa)dimerization reactionb)disporportionation reactionc)condensation reactiond)precipitation reactionCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
PASSAGE - 2There are some deposits of nitrates and phosphates in earth’s crust. Nitrates are more soluble in water. Nitrates are difficult to reduce under the laboratory conditions but microbes do it easily.Ammonia forms large number of complexes with transition metal ions. Hybridization easily explains the ease of sigma donation capability of NH3 and PH3. Phosphine is a flammable gas and is prepared from white phosphorous.Q.White phosphorus on reaction with NaOH gives PH3 as one of the products. This is aa)dimerization reactionb)disporportionation reactionc)condensation reactiond)precipitation reactionCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for PASSAGE - 2There are some deposits of nitrates and phosphates in earth’s crust. Nitrates are more soluble in water. Nitrates are difficult to reduce under the laboratory conditions but microbes do it easily.Ammonia forms large number of complexes with transition metal ions. Hybridization easily explains the ease of sigma donation capability of NH3 and PH3. Phosphine is a flammable gas and is prepared from white phosphorous.Q.White phosphorus on reaction with NaOH gives PH3 as one of the products. This is aa)dimerization reactionb)disporportionation reactionc)condensation reactiond)precipitation reactionCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of PASSAGE - 2There are some deposits of nitrates and phosphates in earth’s crust. Nitrates are more soluble in water. Nitrates are difficult to reduce under the laboratory conditions but microbes do it easily.Ammonia forms large number of complexes with transition metal ions. Hybridization easily explains the ease of sigma donation capability of NH3 and PH3. Phosphine is a flammable gas and is prepared from white phosphorous.Q.White phosphorus on reaction with NaOH gives PH3 as one of the products. This is aa)dimerization reactionb)disporportionation reactionc)condensation reactiond)precipitation reactionCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice PASSAGE - 2There are some deposits of nitrates and phosphates in earth’s crust. Nitrates are more soluble in water. Nitrates are difficult to reduce under the laboratory conditions but microbes do it easily.Ammonia forms large number of complexes with transition metal ions. Hybridization easily explains the ease of sigma donation capability of NH3 and PH3. Phosphine is a flammable gas and is prepared from white phosphorous.Q.White phosphorus on reaction with NaOH gives PH3 as one of the products. This is aa)dimerization reactionb)disporportionation reactionc)condensation reactiond)precipitation reactionCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























