Class 12 Exam > Class 12 Questions > Passage IIWhen compound 1 is heated with C2H5...
Start Learning for Free
Passage II
When compound 1 is heated with C2H5ONa compound 2 and 3 are formed:
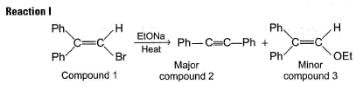
Two mechanisms were proposed for reaction I.
Mechanism A HBr is eliminated from compound 1 to form a symmetrical vinyl carbene intermediate A, which then rearranges to compound 2.
Mechanism B Ethoxide ion first abstract a proton to form a carbanion intermediate B which then rearranges with loss of bromide ion to form compound 2
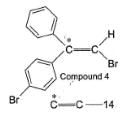
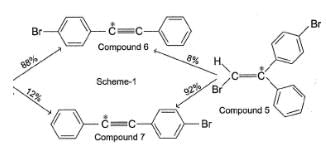
To distinguish between the two machanisms, an isotopic labeling experiment was designed. Two compounds (Compound 4 and 5) were labelled with C-14 and each was treated separately with sodium ethoxide under identical experimental condition where following results were obtained.
Q.
Compound 2 and 6 can be distinguished from each other by all of the following techniques except:
- a)gas chromatography
- b)mass spectrometry
- c)dipole moment measurement
- d)polarimetry
Correct answer is option 'D'. Can you explain this answer?
Verified Answer
Passage IIWhen compound 1 is heated with C2H5ONa compound 2 and 3 are ...
2 and 6 are different compounds, can be separated by gas chromatography. They have different mass, can be distinguished by mass-spectrometry. 2 and 6 have different polarity, can be distinguished by dipole moment measurement. However both are achiral, cannot be distinguished by polarimetry.

|
Explore Courses for Class 12 exam
|

|
Question Description
Passage IIWhen compound 1 is heated with C2H5ONa compound 2 and 3 are formed:Two mechanisms were proposed for reaction I.Mechanism A HBr is eliminated from compound 1 to form a symmetrical vinyl carbene intermediate A, which then rearranges to compound 2.Mechanism B Ethoxide ion first abstract a proton to form a carbanion intermediate B which then rearranges with loss of bromide ion to form compound 2To distinguish between the two machanisms, an isotopic labeling experiment was designed. Two compounds (Compound 4 and 5) were labelled with C-14 and each was treated separately with sodium ethoxide under identical experimental condition where following results were obtained.Q.Compound 2 and 6 can be distinguished from each other by all of the following techniques except:a)gas chromatographyb)mass spectrometryc)dipole moment measurementd)polarimetryCorrect answer is option 'D'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Passage IIWhen compound 1 is heated with C2H5ONa compound 2 and 3 are formed:Two mechanisms were proposed for reaction I.Mechanism A HBr is eliminated from compound 1 to form a symmetrical vinyl carbene intermediate A, which then rearranges to compound 2.Mechanism B Ethoxide ion first abstract a proton to form a carbanion intermediate B which then rearranges with loss of bromide ion to form compound 2To distinguish between the two machanisms, an isotopic labeling experiment was designed. Two compounds (Compound 4 and 5) were labelled with C-14 and each was treated separately with sodium ethoxide under identical experimental condition where following results were obtained.Q.Compound 2 and 6 can be distinguished from each other by all of the following techniques except:a)gas chromatographyb)mass spectrometryc)dipole moment measurementd)polarimetryCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Passage IIWhen compound 1 is heated with C2H5ONa compound 2 and 3 are formed:Two mechanisms were proposed for reaction I.Mechanism A HBr is eliminated from compound 1 to form a symmetrical vinyl carbene intermediate A, which then rearranges to compound 2.Mechanism B Ethoxide ion first abstract a proton to form a carbanion intermediate B which then rearranges with loss of bromide ion to form compound 2To distinguish between the two machanisms, an isotopic labeling experiment was designed. Two compounds (Compound 4 and 5) were labelled with C-14 and each was treated separately with sodium ethoxide under identical experimental condition where following results were obtained.Q.Compound 2 and 6 can be distinguished from each other by all of the following techniques except:a)gas chromatographyb)mass spectrometryc)dipole moment measurementd)polarimetryCorrect answer is option 'D'. Can you explain this answer?.
Passage IIWhen compound 1 is heated with C2H5ONa compound 2 and 3 are formed:Two mechanisms were proposed for reaction I.Mechanism A HBr is eliminated from compound 1 to form a symmetrical vinyl carbene intermediate A, which then rearranges to compound 2.Mechanism B Ethoxide ion first abstract a proton to form a carbanion intermediate B which then rearranges with loss of bromide ion to form compound 2To distinguish between the two machanisms, an isotopic labeling experiment was designed. Two compounds (Compound 4 and 5) were labelled with C-14 and each was treated separately with sodium ethoxide under identical experimental condition where following results were obtained.Q.Compound 2 and 6 can be distinguished from each other by all of the following techniques except:a)gas chromatographyb)mass spectrometryc)dipole moment measurementd)polarimetryCorrect answer is option 'D'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Passage IIWhen compound 1 is heated with C2H5ONa compound 2 and 3 are formed:Two mechanisms were proposed for reaction I.Mechanism A HBr is eliminated from compound 1 to form a symmetrical vinyl carbene intermediate A, which then rearranges to compound 2.Mechanism B Ethoxide ion first abstract a proton to form a carbanion intermediate B which then rearranges with loss of bromide ion to form compound 2To distinguish between the two machanisms, an isotopic labeling experiment was designed. Two compounds (Compound 4 and 5) were labelled with C-14 and each was treated separately with sodium ethoxide under identical experimental condition where following results were obtained.Q.Compound 2 and 6 can be distinguished from each other by all of the following techniques except:a)gas chromatographyb)mass spectrometryc)dipole moment measurementd)polarimetryCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Passage IIWhen compound 1 is heated with C2H5ONa compound 2 and 3 are formed:Two mechanisms were proposed for reaction I.Mechanism A HBr is eliminated from compound 1 to form a symmetrical vinyl carbene intermediate A, which then rearranges to compound 2.Mechanism B Ethoxide ion first abstract a proton to form a carbanion intermediate B which then rearranges with loss of bromide ion to form compound 2To distinguish between the two machanisms, an isotopic labeling experiment was designed. Two compounds (Compound 4 and 5) were labelled with C-14 and each was treated separately with sodium ethoxide under identical experimental condition where following results were obtained.Q.Compound 2 and 6 can be distinguished from each other by all of the following techniques except:a)gas chromatographyb)mass spectrometryc)dipole moment measurementd)polarimetryCorrect answer is option 'D'. Can you explain this answer?.
Solutions for Passage IIWhen compound 1 is heated with C2H5ONa compound 2 and 3 are formed:Two mechanisms were proposed for reaction I.Mechanism A HBr is eliminated from compound 1 to form a symmetrical vinyl carbene intermediate A, which then rearranges to compound 2.Mechanism B Ethoxide ion first abstract a proton to form a carbanion intermediate B which then rearranges with loss of bromide ion to form compound 2To distinguish between the two machanisms, an isotopic labeling experiment was designed. Two compounds (Compound 4 and 5) were labelled with C-14 and each was treated separately with sodium ethoxide under identical experimental condition where following results were obtained.Q.Compound 2 and 6 can be distinguished from each other by all of the following techniques except:a)gas chromatographyb)mass spectrometryc)dipole moment measurementd)polarimetryCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Passage IIWhen compound 1 is heated with C2H5ONa compound 2 and 3 are formed:Two mechanisms were proposed for reaction I.Mechanism A HBr is eliminated from compound 1 to form a symmetrical vinyl carbene intermediate A, which then rearranges to compound 2.Mechanism B Ethoxide ion first abstract a proton to form a carbanion intermediate B which then rearranges with loss of bromide ion to form compound 2To distinguish between the two machanisms, an isotopic labeling experiment was designed. Two compounds (Compound 4 and 5) were labelled with C-14 and each was treated separately with sodium ethoxide under identical experimental condition where following results were obtained.Q.Compound 2 and 6 can be distinguished from each other by all of the following techniques except:a)gas chromatographyb)mass spectrometryc)dipole moment measurementd)polarimetryCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Passage IIWhen compound 1 is heated with C2H5ONa compound 2 and 3 are formed:Two mechanisms were proposed for reaction I.Mechanism A HBr is eliminated from compound 1 to form a symmetrical vinyl carbene intermediate A, which then rearranges to compound 2.Mechanism B Ethoxide ion first abstract a proton to form a carbanion intermediate B which then rearranges with loss of bromide ion to form compound 2To distinguish between the two machanisms, an isotopic labeling experiment was designed. Two compounds (Compound 4 and 5) were labelled with C-14 and each was treated separately with sodium ethoxide under identical experimental condition where following results were obtained.Q.Compound 2 and 6 can be distinguished from each other by all of the following techniques except:a)gas chromatographyb)mass spectrometryc)dipole moment measurementd)polarimetryCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for Passage IIWhen compound 1 is heated with C2H5ONa compound 2 and 3 are formed:Two mechanisms were proposed for reaction I.Mechanism A HBr is eliminated from compound 1 to form a symmetrical vinyl carbene intermediate A, which then rearranges to compound 2.Mechanism B Ethoxide ion first abstract a proton to form a carbanion intermediate B which then rearranges with loss of bromide ion to form compound 2To distinguish between the two machanisms, an isotopic labeling experiment was designed. Two compounds (Compound 4 and 5) were labelled with C-14 and each was treated separately with sodium ethoxide under identical experimental condition where following results were obtained.Q.Compound 2 and 6 can be distinguished from each other by all of the following techniques except:a)gas chromatographyb)mass spectrometryc)dipole moment measurementd)polarimetryCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of Passage IIWhen compound 1 is heated with C2H5ONa compound 2 and 3 are formed:Two mechanisms were proposed for reaction I.Mechanism A HBr is eliminated from compound 1 to form a symmetrical vinyl carbene intermediate A, which then rearranges to compound 2.Mechanism B Ethoxide ion first abstract a proton to form a carbanion intermediate B which then rearranges with loss of bromide ion to form compound 2To distinguish between the two machanisms, an isotopic labeling experiment was designed. Two compounds (Compound 4 and 5) were labelled with C-14 and each was treated separately with sodium ethoxide under identical experimental condition where following results were obtained.Q.Compound 2 and 6 can be distinguished from each other by all of the following techniques except:a)gas chromatographyb)mass spectrometryc)dipole moment measurementd)polarimetryCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Passage IIWhen compound 1 is heated with C2H5ONa compound 2 and 3 are formed:Two mechanisms were proposed for reaction I.Mechanism A HBr is eliminated from compound 1 to form a symmetrical vinyl carbene intermediate A, which then rearranges to compound 2.Mechanism B Ethoxide ion first abstract a proton to form a carbanion intermediate B which then rearranges with loss of bromide ion to form compound 2To distinguish between the two machanisms, an isotopic labeling experiment was designed. Two compounds (Compound 4 and 5) were labelled with C-14 and each was treated separately with sodium ethoxide under identical experimental condition where following results were obtained.Q.Compound 2 and 6 can be distinguished from each other by all of the following techniques except:a)gas chromatographyb)mass spectrometryc)dipole moment measurementd)polarimetryCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























