JEE Exam > JEE Questions > Copper ore is heated in a blast furnace after...
Start Learning for Free
Copper ore is heated in a blast furnace after mixing with silica. Iron oxide slags off as iron silicate and copper is produced in the form of
- a)Copper sulphate
- b)Copper Silicate
- c)Copper matte
- d)Copper hydroxide
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
Copper ore is heated in a blast furnace after mixing with silica. Iron...
During smelting, the roasted ore is mixed with coke and silica in a small blast furnace. The changes occurring are
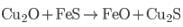
Most of
FeS is oxidised to ferrous oxide
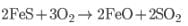
FeO removed as slag
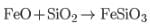 (slag) at the lowest point of furnace, motion mass is Cu2S plus little FeS.
(slag) at the lowest point of furnace, motion mass is Cu2S plus little FeS.
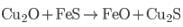
Most of
FeS is oxidised to ferrous oxide
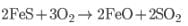
FeO removed as slag
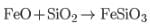 (slag) at the lowest point of furnace, motion mass is Cu2S plus little FeS.
(slag) at the lowest point of furnace, motion mass is Cu2S plus little FeS.Most Upvoted Answer
Copper ore is heated in a blast furnace after mixing with silica. Iron...
Copper ore is heated in a blast furnace after mixing with silica to extract copper. During this process, several reactions occur which result in the production of copper in the form of copper matte.
1. Reaction between Copper Ore and Silica:
The copper ore, which usually contains copper sulfide, is mixed with silica (SiO2) in the blast furnace. The silica acts as a flux, helping to remove impurities and facilitating the formation of slag. The main reaction that takes place is:
CuFeS2 + 2SiO2 → Cu2S + 2FeSiO3
In this reaction, copper sulfide (CuFeS2) reacts with silica to form copper(I) sulfide (Cu2S) and iron silicate (FeSiO3). The iron silicate is the slag that is formed and separates from the molten copper.
2. Formation of Copper Matte:
The copper(I) sulfide (Cu2S) produced in the previous reaction is further heated in the blast furnace. At high temperatures, the copper(I) sulfide reacts with more copper(I) sulfide to form copper(I) matte, which is a mixture of copper(I) sulfide and some copper(I) oxide:
2Cu2S + O2 → 2Cu2O + 2SO2
3Cu2S + 2Cu2O → 6Cu2S + SO2
The copper matte is a black, brittle material that contains a high concentration of copper. It is tapped from the blast furnace and further processed to obtain pure copper.
3. Conversion to Copper Metal:
The copper matte is then subjected to a process called smelting, where it is heated in the presence of oxygen. The oxygen reacts with the impurities in the matte, such as iron and sulfur, to form slag. The remaining copper is then further refined to remove any remaining impurities and obtain pure copper metal.
In summary, when copper ore is heated in a blast furnace after mixing with silica, the iron oxide slags off as iron silicate, and copper is produced in the form of copper matte. This copper matte is then processed to obtain pure copper metal.
1. Reaction between Copper Ore and Silica:
The copper ore, which usually contains copper sulfide, is mixed with silica (SiO2) in the blast furnace. The silica acts as a flux, helping to remove impurities and facilitating the formation of slag. The main reaction that takes place is:
CuFeS2 + 2SiO2 → Cu2S + 2FeSiO3
In this reaction, copper sulfide (CuFeS2) reacts with silica to form copper(I) sulfide (Cu2S) and iron silicate (FeSiO3). The iron silicate is the slag that is formed and separates from the molten copper.
2. Formation of Copper Matte:
The copper(I) sulfide (Cu2S) produced in the previous reaction is further heated in the blast furnace. At high temperatures, the copper(I) sulfide reacts with more copper(I) sulfide to form copper(I) matte, which is a mixture of copper(I) sulfide and some copper(I) oxide:
2Cu2S + O2 → 2Cu2O + 2SO2
3Cu2S + 2Cu2O → 6Cu2S + SO2
The copper matte is a black, brittle material that contains a high concentration of copper. It is tapped from the blast furnace and further processed to obtain pure copper.
3. Conversion to Copper Metal:
The copper matte is then subjected to a process called smelting, where it is heated in the presence of oxygen. The oxygen reacts with the impurities in the matte, such as iron and sulfur, to form slag. The remaining copper is then further refined to remove any remaining impurities and obtain pure copper metal.
In summary, when copper ore is heated in a blast furnace after mixing with silica, the iron oxide slags off as iron silicate, and copper is produced in the form of copper matte. This copper matte is then processed to obtain pure copper metal.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
Copper ore is heated in a blast furnace after mixing with silica. Iron oxide slags off as iron silicate and copper is produced in the form ofa)Copper sulphateb)Copper Silicatec)Copper matted)Copper hydroxideCorrect answer is option 'C'. Can you explain this answer?
Question Description
Copper ore is heated in a blast furnace after mixing with silica. Iron oxide slags off as iron silicate and copper is produced in the form ofa)Copper sulphateb)Copper Silicatec)Copper matted)Copper hydroxideCorrect answer is option 'C'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Copper ore is heated in a blast furnace after mixing with silica. Iron oxide slags off as iron silicate and copper is produced in the form ofa)Copper sulphateb)Copper Silicatec)Copper matted)Copper hydroxideCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Copper ore is heated in a blast furnace after mixing with silica. Iron oxide slags off as iron silicate and copper is produced in the form ofa)Copper sulphateb)Copper Silicatec)Copper matted)Copper hydroxideCorrect answer is option 'C'. Can you explain this answer?.
Copper ore is heated in a blast furnace after mixing with silica. Iron oxide slags off as iron silicate and copper is produced in the form ofa)Copper sulphateb)Copper Silicatec)Copper matted)Copper hydroxideCorrect answer is option 'C'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Copper ore is heated in a blast furnace after mixing with silica. Iron oxide slags off as iron silicate and copper is produced in the form ofa)Copper sulphateb)Copper Silicatec)Copper matted)Copper hydroxideCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Copper ore is heated in a blast furnace after mixing with silica. Iron oxide slags off as iron silicate and copper is produced in the form ofa)Copper sulphateb)Copper Silicatec)Copper matted)Copper hydroxideCorrect answer is option 'C'. Can you explain this answer?.
Solutions for Copper ore is heated in a blast furnace after mixing with silica. Iron oxide slags off as iron silicate and copper is produced in the form ofa)Copper sulphateb)Copper Silicatec)Copper matted)Copper hydroxideCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Copper ore is heated in a blast furnace after mixing with silica. Iron oxide slags off as iron silicate and copper is produced in the form ofa)Copper sulphateb)Copper Silicatec)Copper matted)Copper hydroxideCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Copper ore is heated in a blast furnace after mixing with silica. Iron oxide slags off as iron silicate and copper is produced in the form ofa)Copper sulphateb)Copper Silicatec)Copper matted)Copper hydroxideCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for Copper ore is heated in a blast furnace after mixing with silica. Iron oxide slags off as iron silicate and copper is produced in the form ofa)Copper sulphateb)Copper Silicatec)Copper matted)Copper hydroxideCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of Copper ore is heated in a blast furnace after mixing with silica. Iron oxide slags off as iron silicate and copper is produced in the form ofa)Copper sulphateb)Copper Silicatec)Copper matted)Copper hydroxideCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Copper ore is heated in a blast furnace after mixing with silica. Iron oxide slags off as iron silicate and copper is produced in the form ofa)Copper sulphateb)Copper Silicatec)Copper matted)Copper hydroxideCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


























