Class 12 Exam > Class 12 Questions > In naming complexes, cation is named first fo...
Start Learning for Free
In naming complexes, cation is named first followed by anion. Non-ionic or neutral complexes are given a one word name. The cationic and neutral complex ions are named by writing ‘the number and name of the ligands followed by the name of the central metal ion with its oxidation number in roman numerals within the parenthesis. If the complex is anionic, name of the metals ends with ‘ate’. When the complex contain two or more metal atoms linked with the help of bridging ligand then each bridging ligand is started with μ.
Q.
The IUPAC name of [Ni(NH3)4] [NiCI4] is
- a)tetrachloronickel (II) tetramminenickel (II)
- b)tetramminenickel (II) tetrachloronickel (II)
- c)tetramminenickel (II) tetrachloronickelate (II)
- d)tetrachloronickel (II) tetramminenickelate (0)
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
In naming complexes, cation is named first followed by anion. Non-ioni...
The IUPAC name of [Ni(NH3)4][NiCI4] is tetramminenickel (II) tetrachloronickelate (II).
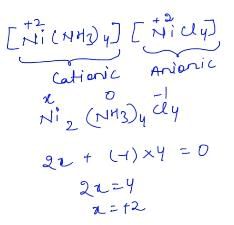
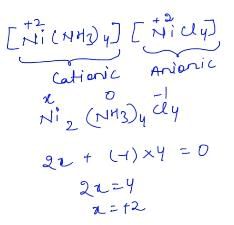
Most Upvoted Answer
In naming complexes, cation is named first followed by anion. Non-ioni...
Understanding the Complex: [Ni(NH3)4][NiCl4]
To derive the IUPAC name for the complex, we need to analyze its components:
1. Identifying the Cation and Anion
- The complex is made up of two parts:
- Cation: [Ni(NH3)4]
- Anion: [NiCl4]
2. Naming the Cation
- The cation contains four ammonia (NH3) ligands.
- The name for ammonia as a ligand is "ammine."
- Nickel in this cation has an oxidation state of +2 (since there are no charges on NH3).
- Therefore, the name for the cation is "tetramminenickel (II)."
3. Naming the Anion
- The anion contains four chloride (Cl) ligands.
- When naming the anion, the metal name "nickel" changes to "nickelate" to reflect its anionic nature.
- Nickel also has an oxidation state of +2 in the anion.
- Thus, the name for the anion is "tetrachloronickelate (II)."
4. Combining the Names
- According to IUPAC naming conventions, the cation is named first, followed by the anion.
- Therefore, the full IUPAC name for the complex [Ni(NH3)4][NiCl4] is:
tetramminenickel (II) tetrachloronickelate (II)
Final Answer
- The correct option is c) tetramminenickel (II) tetrachloronickelate (II).
This systematic approach to naming ensures clarity and adherence to chemical nomenclature rules.
To derive the IUPAC name for the complex, we need to analyze its components:
1. Identifying the Cation and Anion
- The complex is made up of two parts:
- Cation: [Ni(NH3)4]
- Anion: [NiCl4]
2. Naming the Cation
- The cation contains four ammonia (NH3) ligands.
- The name for ammonia as a ligand is "ammine."
- Nickel in this cation has an oxidation state of +2 (since there are no charges on NH3).
- Therefore, the name for the cation is "tetramminenickel (II)."
3. Naming the Anion
- The anion contains four chloride (Cl) ligands.
- When naming the anion, the metal name "nickel" changes to "nickelate" to reflect its anionic nature.
- Nickel also has an oxidation state of +2 in the anion.
- Thus, the name for the anion is "tetrachloronickelate (II)."
4. Combining the Names
- According to IUPAC naming conventions, the cation is named first, followed by the anion.
- Therefore, the full IUPAC name for the complex [Ni(NH3)4][NiCl4] is:
tetramminenickel (II) tetrachloronickelate (II)
Final Answer
- The correct option is c) tetramminenickel (II) tetrachloronickelate (II).
This systematic approach to naming ensures clarity and adherence to chemical nomenclature rules.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
Question Description
In naming complexes, cation is named first followed by anion. Non-ionic or neutral complexes are given a one word name. The cationic and neutral complex ions are named by writing ‘the number and name of the ligands followed by the name of the central metal ion with its oxidation number in roman numerals within the parenthesis. If the complex is anionic, name of the metals ends with ‘ate’. When the complex contain two or more metal atoms linked with the help of bridging ligand then each bridging ligand is started with μ.Q.The IUPAC name of [Ni(NH3)4] [NiCI4] isa)tetrachloronickel (II) tetramminenickel (II)b)tetramminenickel (II) tetrachloronickel (II)c)tetramminenickel (II) tetrachloronickelate (II)d)tetrachloronickel (II) tetramminenickelate (0)Correct answer is option 'C'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about In naming complexes, cation is named first followed by anion. Non-ionic or neutral complexes are given a one word name. The cationic and neutral complex ions are named by writing ‘the number and name of the ligands followed by the name of the central metal ion with its oxidation number in roman numerals within the parenthesis. If the complex is anionic, name of the metals ends with ‘ate’. When the complex contain two or more metal atoms linked with the help of bridging ligand then each bridging ligand is started with μ.Q.The IUPAC name of [Ni(NH3)4] [NiCI4] isa)tetrachloronickel (II) tetramminenickel (II)b)tetramminenickel (II) tetrachloronickel (II)c)tetramminenickel (II) tetrachloronickelate (II)d)tetrachloronickel (II) tetramminenickelate (0)Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In naming complexes, cation is named first followed by anion. Non-ionic or neutral complexes are given a one word name. The cationic and neutral complex ions are named by writing ‘the number and name of the ligands followed by the name of the central metal ion with its oxidation number in roman numerals within the parenthesis. If the complex is anionic, name of the metals ends with ‘ate’. When the complex contain two or more metal atoms linked with the help of bridging ligand then each bridging ligand is started with μ.Q.The IUPAC name of [Ni(NH3)4] [NiCI4] isa)tetrachloronickel (II) tetramminenickel (II)b)tetramminenickel (II) tetrachloronickel (II)c)tetramminenickel (II) tetrachloronickelate (II)d)tetrachloronickel (II) tetramminenickelate (0)Correct answer is option 'C'. Can you explain this answer?.
In naming complexes, cation is named first followed by anion. Non-ionic or neutral complexes are given a one word name. The cationic and neutral complex ions are named by writing ‘the number and name of the ligands followed by the name of the central metal ion with its oxidation number in roman numerals within the parenthesis. If the complex is anionic, name of the metals ends with ‘ate’. When the complex contain two or more metal atoms linked with the help of bridging ligand then each bridging ligand is started with μ.Q.The IUPAC name of [Ni(NH3)4] [NiCI4] isa)tetrachloronickel (II) tetramminenickel (II)b)tetramminenickel (II) tetrachloronickel (II)c)tetramminenickel (II) tetrachloronickelate (II)d)tetrachloronickel (II) tetramminenickelate (0)Correct answer is option 'C'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about In naming complexes, cation is named first followed by anion. Non-ionic or neutral complexes are given a one word name. The cationic and neutral complex ions are named by writing ‘the number and name of the ligands followed by the name of the central metal ion with its oxidation number in roman numerals within the parenthesis. If the complex is anionic, name of the metals ends with ‘ate’. When the complex contain two or more metal atoms linked with the help of bridging ligand then each bridging ligand is started with μ.Q.The IUPAC name of [Ni(NH3)4] [NiCI4] isa)tetrachloronickel (II) tetramminenickel (II)b)tetramminenickel (II) tetrachloronickel (II)c)tetramminenickel (II) tetrachloronickelate (II)d)tetrachloronickel (II) tetramminenickelate (0)Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In naming complexes, cation is named first followed by anion. Non-ionic or neutral complexes are given a one word name. The cationic and neutral complex ions are named by writing ‘the number and name of the ligands followed by the name of the central metal ion with its oxidation number in roman numerals within the parenthesis. If the complex is anionic, name of the metals ends with ‘ate’. When the complex contain two or more metal atoms linked with the help of bridging ligand then each bridging ligand is started with μ.Q.The IUPAC name of [Ni(NH3)4] [NiCI4] isa)tetrachloronickel (II) tetramminenickel (II)b)tetramminenickel (II) tetrachloronickel (II)c)tetramminenickel (II) tetrachloronickelate (II)d)tetrachloronickel (II) tetramminenickelate (0)Correct answer is option 'C'. Can you explain this answer?.
Solutions for In naming complexes, cation is named first followed by anion. Non-ionic or neutral complexes are given a one word name. The cationic and neutral complex ions are named by writing ‘the number and name of the ligands followed by the name of the central metal ion with its oxidation number in roman numerals within the parenthesis. If the complex is anionic, name of the metals ends with ‘ate’. When the complex contain two or more metal atoms linked with the help of bridging ligand then each bridging ligand is started with μ.Q.The IUPAC name of [Ni(NH3)4] [NiCI4] isa)tetrachloronickel (II) tetramminenickel (II)b)tetramminenickel (II) tetrachloronickel (II)c)tetramminenickel (II) tetrachloronickelate (II)d)tetrachloronickel (II) tetramminenickelate (0)Correct answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of In naming complexes, cation is named first followed by anion. Non-ionic or neutral complexes are given a one word name. The cationic and neutral complex ions are named by writing ‘the number and name of the ligands followed by the name of the central metal ion with its oxidation number in roman numerals within the parenthesis. If the complex is anionic, name of the metals ends with ‘ate’. When the complex contain two or more metal atoms linked with the help of bridging ligand then each bridging ligand is started with μ.Q.The IUPAC name of [Ni(NH3)4] [NiCI4] isa)tetrachloronickel (II) tetramminenickel (II)b)tetramminenickel (II) tetrachloronickel (II)c)tetramminenickel (II) tetrachloronickelate (II)d)tetrachloronickel (II) tetramminenickelate (0)Correct answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
In naming complexes, cation is named first followed by anion. Non-ionic or neutral complexes are given a one word name. The cationic and neutral complex ions are named by writing ‘the number and name of the ligands followed by the name of the central metal ion with its oxidation number in roman numerals within the parenthesis. If the complex is anionic, name of the metals ends with ‘ate’. When the complex contain two or more metal atoms linked with the help of bridging ligand then each bridging ligand is started with μ.Q.The IUPAC name of [Ni(NH3)4] [NiCI4] isa)tetrachloronickel (II) tetramminenickel (II)b)tetramminenickel (II) tetrachloronickel (II)c)tetramminenickel (II) tetrachloronickelate (II)d)tetrachloronickel (II) tetramminenickelate (0)Correct answer is option 'C'. Can you explain this answer?, a detailed solution for In naming complexes, cation is named first followed by anion. Non-ionic or neutral complexes are given a one word name. The cationic and neutral complex ions are named by writing ‘the number and name of the ligands followed by the name of the central metal ion with its oxidation number in roman numerals within the parenthesis. If the complex is anionic, name of the metals ends with ‘ate’. When the complex contain two or more metal atoms linked with the help of bridging ligand then each bridging ligand is started with μ.Q.The IUPAC name of [Ni(NH3)4] [NiCI4] isa)tetrachloronickel (II) tetramminenickel (II)b)tetramminenickel (II) tetrachloronickel (II)c)tetramminenickel (II) tetrachloronickelate (II)d)tetrachloronickel (II) tetramminenickelate (0)Correct answer is option 'C'. Can you explain this answer? has been provided alongside types of In naming complexes, cation is named first followed by anion. Non-ionic or neutral complexes are given a one word name. The cationic and neutral complex ions are named by writing ‘the number and name of the ligands followed by the name of the central metal ion with its oxidation number in roman numerals within the parenthesis. If the complex is anionic, name of the metals ends with ‘ate’. When the complex contain two or more metal atoms linked with the help of bridging ligand then each bridging ligand is started with μ.Q.The IUPAC name of [Ni(NH3)4] [NiCI4] isa)tetrachloronickel (II) tetramminenickel (II)b)tetramminenickel (II) tetrachloronickel (II)c)tetramminenickel (II) tetrachloronickelate (II)d)tetrachloronickel (II) tetramminenickelate (0)Correct answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice In naming complexes, cation is named first followed by anion. Non-ionic or neutral complexes are given a one word name. The cationic and neutral complex ions are named by writing ‘the number and name of the ligands followed by the name of the central metal ion with its oxidation number in roman numerals within the parenthesis. If the complex is anionic, name of the metals ends with ‘ate’. When the complex contain two or more metal atoms linked with the help of bridging ligand then each bridging ligand is started with μ.Q.The IUPAC name of [Ni(NH3)4] [NiCI4] isa)tetrachloronickel (II) tetramminenickel (II)b)tetramminenickel (II) tetrachloronickel (II)c)tetramminenickel (II) tetrachloronickelate (II)d)tetrachloronickel (II) tetramminenickelate (0)Correct answer is option 'C'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup to solve all Doubts
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.





















