Class 12 Exam > Class 12 Questions > Direction (Q. Nos. 16and 17) This section con...
Start Learning for Free
Direction (Q. Nos. 16 and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
A salt A when heated with K2Cr2O7 and cone. H2SO4 liberates a gas which is absorbed in NaOH solution. The NaOH solution turns yellow. When this solution is acidified with acetic acid and lead acetate solution is added, a yellow precipitate B is formed. When A is mixed with MnO2 and heated with cone. H2SO4 , a gas C is evolved which turns a starch-iodide paper blue.
Q.
What is the colour of the gas which is evolved when salt A is heated with MnO2 and H2SO4?
- a)Violet
- b)Brown
- c)Greenish yellow
- d)Colourless
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
Direction (Q. Nos. 16and 17) This section contains a paragraph, descri...
Greenish yellow gas is chlorine.
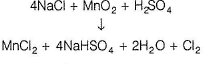
Most Upvoted Answer
Direction (Q. Nos. 16and 17) This section contains a paragraph, descri...
Colour of the gas evolved when salt A is heated with MnO2 and H2SO4 is greenish yellow.
Explanation:
When salt A is heated with MnO2 and H2SO4, a gas C is evolved. This gas turns a starch-iodide paper blue. The blue coloration of the starch-iodide paper indicates the presence of iodine.
Reactions:
The reaction between salt A and MnO2 can be represented as follows:
2A + MnO2 + 2H2SO4 → 2ASO4 + MnSO4 + 2H2O + C(gas)
The gas C evolved in the above reaction is greenish yellow in color.
Explanation of the reaction:
1. The presence of MnO2 and H2SO4 in the reaction mixture provides the necessary conditions for the oxidation of A.
2. As a result of this oxidation, gas C is evolved.
3. The gas C is likely to be chlorine (Cl2) or sulfur dioxide (SO2).
4. Chlorine gas is greenish yellow in color, whereas sulfur dioxide is colorless.
5. Since the gas C turns a starch-iodide paper blue, it confirms the presence of iodine in the gas.
6. Chlorine gas reacts with iodide ions present in the starch-iodide paper to form iodine, which imparts a blue coloration.
Conclusion:
Hence, the gas evolved when salt A is heated with MnO2 and H2SO4 is greenish yellow in color.
Explanation:
When salt A is heated with MnO2 and H2SO4, a gas C is evolved. This gas turns a starch-iodide paper blue. The blue coloration of the starch-iodide paper indicates the presence of iodine.
Reactions:
The reaction between salt A and MnO2 can be represented as follows:
2A + MnO2 + 2H2SO4 → 2ASO4 + MnSO4 + 2H2O + C(gas)
The gas C evolved in the above reaction is greenish yellow in color.
Explanation of the reaction:
1. The presence of MnO2 and H2SO4 in the reaction mixture provides the necessary conditions for the oxidation of A.
2. As a result of this oxidation, gas C is evolved.
3. The gas C is likely to be chlorine (Cl2) or sulfur dioxide (SO2).
4. Chlorine gas is greenish yellow in color, whereas sulfur dioxide is colorless.
5. Since the gas C turns a starch-iodide paper blue, it confirms the presence of iodine in the gas.
6. Chlorine gas reacts with iodide ions present in the starch-iodide paper to form iodine, which imparts a blue coloration.
Conclusion:
Hence, the gas evolved when salt A is heated with MnO2 and H2SO4 is greenish yellow in color.

|
Explore Courses for Class 12 exam
|

|
Question Description
Direction (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageA salt A when heated with K2Cr2O7 and cone. H2SO4 liberates a gas which is absorbed in NaOH solution. The NaOH solution turns yellow. When this solution is acidified with acetic acid and lead acetate solution is added, a yellow precipitate B is formed. When A is mixed with MnO2 and heated with cone. H2SO4 , a gas C is evolved which turns a starch-iodide paper blue.Q.What is the colour of the gas which is evolved when salt A is heated with MnO2 and H2SO4?a)Violetb)Brownc)Greenish yellowd)ColourlessCorrect answer is option 'C'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Direction (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageA salt A when heated with K2Cr2O7 and cone. H2SO4 liberates a gas which is absorbed in NaOH solution. The NaOH solution turns yellow. When this solution is acidified with acetic acid and lead acetate solution is added, a yellow precipitate B is formed. When A is mixed with MnO2 and heated with cone. H2SO4 , a gas C is evolved which turns a starch-iodide paper blue.Q.What is the colour of the gas which is evolved when salt A is heated with MnO2 and H2SO4?a)Violetb)Brownc)Greenish yellowd)ColourlessCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Direction (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageA salt A when heated with K2Cr2O7 and cone. H2SO4 liberates a gas which is absorbed in NaOH solution. The NaOH solution turns yellow. When this solution is acidified with acetic acid and lead acetate solution is added, a yellow precipitate B is formed. When A is mixed with MnO2 and heated with cone. H2SO4 , a gas C is evolved which turns a starch-iodide paper blue.Q.What is the colour of the gas which is evolved when salt A is heated with MnO2 and H2SO4?a)Violetb)Brownc)Greenish yellowd)ColourlessCorrect answer is option 'C'. Can you explain this answer?.
Direction (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageA salt A when heated with K2Cr2O7 and cone. H2SO4 liberates a gas which is absorbed in NaOH solution. The NaOH solution turns yellow. When this solution is acidified with acetic acid and lead acetate solution is added, a yellow precipitate B is formed. When A is mixed with MnO2 and heated with cone. H2SO4 , a gas C is evolved which turns a starch-iodide paper blue.Q.What is the colour of the gas which is evolved when salt A is heated with MnO2 and H2SO4?a)Violetb)Brownc)Greenish yellowd)ColourlessCorrect answer is option 'C'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Direction (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageA salt A when heated with K2Cr2O7 and cone. H2SO4 liberates a gas which is absorbed in NaOH solution. The NaOH solution turns yellow. When this solution is acidified with acetic acid and lead acetate solution is added, a yellow precipitate B is formed. When A is mixed with MnO2 and heated with cone. H2SO4 , a gas C is evolved which turns a starch-iodide paper blue.Q.What is the colour of the gas which is evolved when salt A is heated with MnO2 and H2SO4?a)Violetb)Brownc)Greenish yellowd)ColourlessCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Direction (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageA salt A when heated with K2Cr2O7 and cone. H2SO4 liberates a gas which is absorbed in NaOH solution. The NaOH solution turns yellow. When this solution is acidified with acetic acid and lead acetate solution is added, a yellow precipitate B is formed. When A is mixed with MnO2 and heated with cone. H2SO4 , a gas C is evolved which turns a starch-iodide paper blue.Q.What is the colour of the gas which is evolved when salt A is heated with MnO2 and H2SO4?a)Violetb)Brownc)Greenish yellowd)ColourlessCorrect answer is option 'C'. Can you explain this answer?.
Solutions for Direction (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageA salt A when heated with K2Cr2O7 and cone. H2SO4 liberates a gas which is absorbed in NaOH solution. The NaOH solution turns yellow. When this solution is acidified with acetic acid and lead acetate solution is added, a yellow precipitate B is formed. When A is mixed with MnO2 and heated with cone. H2SO4 , a gas C is evolved which turns a starch-iodide paper blue.Q.What is the colour of the gas which is evolved when salt A is heated with MnO2 and H2SO4?a)Violetb)Brownc)Greenish yellowd)ColourlessCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Direction (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageA salt A when heated with K2Cr2O7 and cone. H2SO4 liberates a gas which is absorbed in NaOH solution. The NaOH solution turns yellow. When this solution is acidified with acetic acid and lead acetate solution is added, a yellow precipitate B is formed. When A is mixed with MnO2 and heated with cone. H2SO4 , a gas C is evolved which turns a starch-iodide paper blue.Q.What is the colour of the gas which is evolved when salt A is heated with MnO2 and H2SO4?a)Violetb)Brownc)Greenish yellowd)ColourlessCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Direction (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageA salt A when heated with K2Cr2O7 and cone. H2SO4 liberates a gas which is absorbed in NaOH solution. The NaOH solution turns yellow. When this solution is acidified with acetic acid and lead acetate solution is added, a yellow precipitate B is formed. When A is mixed with MnO2 and heated with cone. H2SO4 , a gas C is evolved which turns a starch-iodide paper blue.Q.What is the colour of the gas which is evolved when salt A is heated with MnO2 and H2SO4?a)Violetb)Brownc)Greenish yellowd)ColourlessCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for Direction (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageA salt A when heated with K2Cr2O7 and cone. H2SO4 liberates a gas which is absorbed in NaOH solution. The NaOH solution turns yellow. When this solution is acidified with acetic acid and lead acetate solution is added, a yellow precipitate B is formed. When A is mixed with MnO2 and heated with cone. H2SO4 , a gas C is evolved which turns a starch-iodide paper blue.Q.What is the colour of the gas which is evolved when salt A is heated with MnO2 and H2SO4?a)Violetb)Brownc)Greenish yellowd)ColourlessCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of Direction (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageA salt A when heated with K2Cr2O7 and cone. H2SO4 liberates a gas which is absorbed in NaOH solution. The NaOH solution turns yellow. When this solution is acidified with acetic acid and lead acetate solution is added, a yellow precipitate B is formed. When A is mixed with MnO2 and heated with cone. H2SO4 , a gas C is evolved which turns a starch-iodide paper blue.Q.What is the colour of the gas which is evolved when salt A is heated with MnO2 and H2SO4?a)Violetb)Brownc)Greenish yellowd)ColourlessCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Direction (Q. Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageA salt A when heated with K2Cr2O7 and cone. H2SO4 liberates a gas which is absorbed in NaOH solution. The NaOH solution turns yellow. When this solution is acidified with acetic acid and lead acetate solution is added, a yellow precipitate B is formed. When A is mixed with MnO2 and heated with cone. H2SO4 , a gas C is evolved which turns a starch-iodide paper blue.Q.What is the colour of the gas which is evolved when salt A is heated with MnO2 and H2SO4?a)Violetb)Brownc)Greenish yellowd)ColourlessCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















