Class 12 Exam > Class 12 Questions > Charring of cane sugar by sulphuric acid show...
Start Learning for Free
Charring of cane sugar by sulphuric acid shows ...... property of the acid.
- a)oxidation
- b)complex formation
- c)dehydrating
- d)acidic
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
Charring of cane sugar by sulphuric acid shows ...... property of the ...
Cone. H2SO4 is a strong dehydrating agent. Many wet gases can be dried by passing them through sulphuric acid. H2SO4 removes water from organic compounds. It is evident by its charring action on carbohydrates.
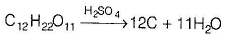
Most Upvoted Answer
Charring of cane sugar by sulphuric acid shows ...... property of the ...
Charring of cane sugar by sulphuric acid shows the property of dehydrating.
Sulphuric acid (H2SO4) is a strong acid and a powerful dehydrating agent. When it reacts with cane sugar (sucrose, C12H22O11), it removes water molecules from the sugar molecule, resulting in the formation of a black carbonaceous substance known as "char". This process is commonly referred to as charring.
Explanation:
1. Dehydrating property of sulphuric acid:
Sulphuric acid is highly hygroscopic, meaning it has a strong affinity for water and readily removes water molecules from other substances. In the case of cane sugar, sulphuric acid acts as a dehydrating agent by abstracting water molecules (H2O) from the sugar molecule.
2. Reaction between sulphuric acid and cane sugar:
The reaction between sulphuric acid and cane sugar can be represented as follows:
C12H22O11 + H2SO4 → 12C + 11H2O + H2SO4
In this reaction, sulphuric acid (H2SO4) acts as a catalyst for the dehydration of cane sugar. The acid abstracts water molecules from the sugar molecule, resulting in the formation of carbon (C) and water (H2O) as products. The released water molecules further react with sulphuric acid, creating more sulphuric acid as a byproduct.
3. Formation of char:
The carbon (C) produced during the reaction accumulates as a black residue, giving the charred appearance. This carbonaceous substance is a result of the incomplete combustion of the sugar molecule due to dehydration.
4. Significance of the reaction:
The charring of cane sugar by sulphuric acid is often used as a test to identify the presence of sugar in various substances. The formation of char indicates the presence of sugar, as other substances do not undergo charring when treated with sulphuric acid.
In conclusion, the charring of cane sugar by sulphuric acid demonstrates the dehydrating property of the acid. Sulphuric acid acts as a catalyst in removing water molecules from the sugar molecule, resulting in the formation of carbon and water. This reaction is commonly used to identify the presence of sugar in substances.
Sulphuric acid (H2SO4) is a strong acid and a powerful dehydrating agent. When it reacts with cane sugar (sucrose, C12H22O11), it removes water molecules from the sugar molecule, resulting in the formation of a black carbonaceous substance known as "char". This process is commonly referred to as charring.
Explanation:
1. Dehydrating property of sulphuric acid:
Sulphuric acid is highly hygroscopic, meaning it has a strong affinity for water and readily removes water molecules from other substances. In the case of cane sugar, sulphuric acid acts as a dehydrating agent by abstracting water molecules (H2O) from the sugar molecule.
2. Reaction between sulphuric acid and cane sugar:
The reaction between sulphuric acid and cane sugar can be represented as follows:
C12H22O11 + H2SO4 → 12C + 11H2O + H2SO4
In this reaction, sulphuric acid (H2SO4) acts as a catalyst for the dehydration of cane sugar. The acid abstracts water molecules from the sugar molecule, resulting in the formation of carbon (C) and water (H2O) as products. The released water molecules further react with sulphuric acid, creating more sulphuric acid as a byproduct.
3. Formation of char:
The carbon (C) produced during the reaction accumulates as a black residue, giving the charred appearance. This carbonaceous substance is a result of the incomplete combustion of the sugar molecule due to dehydration.
4. Significance of the reaction:
The charring of cane sugar by sulphuric acid is often used as a test to identify the presence of sugar in various substances. The formation of char indicates the presence of sugar, as other substances do not undergo charring when treated with sulphuric acid.
In conclusion, the charring of cane sugar by sulphuric acid demonstrates the dehydrating property of the acid. Sulphuric acid acts as a catalyst in removing water molecules from the sugar molecule, resulting in the formation of carbon and water. This reaction is commonly used to identify the presence of sugar in substances.

|
Explore Courses for Class 12 exam
|

|
Question Description
Charring of cane sugar by sulphuric acid shows ...... property of the acid.a)oxidationb)complex formationc)dehydratingd)acidicCorrect answer is option 'C'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Charring of cane sugar by sulphuric acid shows ...... property of the acid.a)oxidationb)complex formationc)dehydratingd)acidicCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Charring of cane sugar by sulphuric acid shows ...... property of the acid.a)oxidationb)complex formationc)dehydratingd)acidicCorrect answer is option 'C'. Can you explain this answer?.
Charring of cane sugar by sulphuric acid shows ...... property of the acid.a)oxidationb)complex formationc)dehydratingd)acidicCorrect answer is option 'C'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Charring of cane sugar by sulphuric acid shows ...... property of the acid.a)oxidationb)complex formationc)dehydratingd)acidicCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Charring of cane sugar by sulphuric acid shows ...... property of the acid.a)oxidationb)complex formationc)dehydratingd)acidicCorrect answer is option 'C'. Can you explain this answer?.
Solutions for Charring of cane sugar by sulphuric acid shows ...... property of the acid.a)oxidationb)complex formationc)dehydratingd)acidicCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Charring of cane sugar by sulphuric acid shows ...... property of the acid.a)oxidationb)complex formationc)dehydratingd)acidicCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Charring of cane sugar by sulphuric acid shows ...... property of the acid.a)oxidationb)complex formationc)dehydratingd)acidicCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for Charring of cane sugar by sulphuric acid shows ...... property of the acid.a)oxidationb)complex formationc)dehydratingd)acidicCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of Charring of cane sugar by sulphuric acid shows ...... property of the acid.a)oxidationb)complex formationc)dehydratingd)acidicCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Charring of cane sugar by sulphuric acid shows ...... property of the acid.a)oxidationb)complex formationc)dehydratingd)acidicCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















