Class 12 Exam > Class 12 Questions > Regarding ammonia correct statements area)A n...
Start Learning for Free
Regarding ammonia correct statements are
- a)A nitride on hydrolysis produce NH3
- b)Haber’s process of ammonia formation is exothermic
- c)NH3 dissolves AgCI forming a soluble complex
- d)Ammonia is a Lewis base as well as Bronsted-Lowry base
Correct answer is option 'A,B,C,D'. Can you explain this answer?
Verified Answer
Regarding ammonia correct statements area)A nitride onhydrolysis produ...
Metal nitride upon hydrolysis gives ammonia,
As we know Haber's process involves the direct combination of nitrogen and hydrogen with the removal of some amount of heat, i.e. this is exothermic in nature. 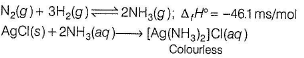
Nitrogen atom of ammonia can donate a lone pair of e- to any species that is why, Lewis base as well as Bronsted-Lowry base.
 This question is part of UPSC exam. View all Class 12 courses
This question is part of UPSC exam. View all Class 12 courses
Most Upvoted Answer
Regarding ammonia correct statements area)A nitride onhydrolysis produ...
A) A nitride on hydrolysis produce NH3:
When a nitride compound is hydrolyzed, it reacts with water to produce ammonia. Nitrides are compounds that contain the nitride ion (N3-), and when they come into contact with water, they undergo hydrolysis. The reaction can be represented as follows:
Nitride (N3-) + Water (H2O) → Ammonia (NH3)
This reaction occurs because the nitride ion reacts with water to form ammonia and hydroxide ions. The ammonia produced is a colorless gas with a pungent odor.
B) Haber's process of ammonia formation is exothermic:
Haber's process, also known as the Haber-Bosch process, is a method for synthesizing ammonia from nitrogen gas (N2) and hydrogen gas (H2). The reaction is catalyzed by an iron catalyst and occurs at high temperatures and pressures. The overall reaction is as follows:
N2(g) + 3H2(g) ⇌ 2NH3(g)
The reaction is exothermic, meaning it releases heat energy. This is because the formation of ammonia from nitrogen and hydrogen is accompanied by a decrease in the total energy of the system.
C) NH3 dissolves AgCl forming a soluble complex:
Ammonia (NH3) is a versatile compound that can act as a ligand and form complexes with metal ions. When ammonia is added to silver chloride (AgCl), it forms a soluble complex called silver diammine chloride. The reaction can be represented as follows:
AgCl(s) + 2NH3(aq) → [Ag(NH3)2]Cl(aq)
In this reaction, the ammonia molecules coordinate with the silver ion (Ag+) and displace the chloride ions (Cl-) from the silver chloride compound. The resulting complex is soluble in water, forming a clear solution.
D) Ammonia is a Lewis base as well as a Bronsted-Lowry base:
Ammonia (NH3) can act as both a Lewis base and a Bronsted-Lowry base. As a Lewis base, ammonia donates a pair of electrons from its lone pair to form a coordinate bond with a Lewis acid. For example, in the reaction with a metal ion like silver (Ag+), ammonia donates its lone pair of electrons to form a coordinate bond with the metal ion.
As a Bronsted-Lowry base, ammonia can accept a proton (H+) to form the ammonium ion (NH4+). In water, ammonia can act as a base by accepting a proton from a water molecule, forming the ammonium ion and hydroxide ion:
NH3(aq) + H2O(l) ⇌ NH4+(aq) + OH-(aq)
Overall, ammonia exhibits both Lewis basicity and Bronsted-Lowry basicity, making it a versatile base in various chemical reactions.
When a nitride compound is hydrolyzed, it reacts with water to produce ammonia. Nitrides are compounds that contain the nitride ion (N3-), and when they come into contact with water, they undergo hydrolysis. The reaction can be represented as follows:
Nitride (N3-) + Water (H2O) → Ammonia (NH3)
This reaction occurs because the nitride ion reacts with water to form ammonia and hydroxide ions. The ammonia produced is a colorless gas with a pungent odor.
B) Haber's process of ammonia formation is exothermic:
Haber's process, also known as the Haber-Bosch process, is a method for synthesizing ammonia from nitrogen gas (N2) and hydrogen gas (H2). The reaction is catalyzed by an iron catalyst and occurs at high temperatures and pressures. The overall reaction is as follows:
N2(g) + 3H2(g) ⇌ 2NH3(g)
The reaction is exothermic, meaning it releases heat energy. This is because the formation of ammonia from nitrogen and hydrogen is accompanied by a decrease in the total energy of the system.
C) NH3 dissolves AgCl forming a soluble complex:
Ammonia (NH3) is a versatile compound that can act as a ligand and form complexes with metal ions. When ammonia is added to silver chloride (AgCl), it forms a soluble complex called silver diammine chloride. The reaction can be represented as follows:
AgCl(s) + 2NH3(aq) → [Ag(NH3)2]Cl(aq)
In this reaction, the ammonia molecules coordinate with the silver ion (Ag+) and displace the chloride ions (Cl-) from the silver chloride compound. The resulting complex is soluble in water, forming a clear solution.
D) Ammonia is a Lewis base as well as a Bronsted-Lowry base:
Ammonia (NH3) can act as both a Lewis base and a Bronsted-Lowry base. As a Lewis base, ammonia donates a pair of electrons from its lone pair to form a coordinate bond with a Lewis acid. For example, in the reaction with a metal ion like silver (Ag+), ammonia donates its lone pair of electrons to form a coordinate bond with the metal ion.
As a Bronsted-Lowry base, ammonia can accept a proton (H+) to form the ammonium ion (NH4+). In water, ammonia can act as a base by accepting a proton from a water molecule, forming the ammonium ion and hydroxide ion:
NH3(aq) + H2O(l) ⇌ NH4+(aq) + OH-(aq)
Overall, ammonia exhibits both Lewis basicity and Bronsted-Lowry basicity, making it a versatile base in various chemical reactions.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
Regarding ammonia correct statements area)A nitride onhydrolysis produce NH3b)Haber’s process of ammonia formation is exothermicc)NH3 dissolves AgCI forming a soluble complexd)Ammonia is a Lewis base as well as Bronsted-Lowry baseCorrect answer is option 'A,B,C,D'. Can you explain this answer?
Question Description
Regarding ammonia correct statements area)A nitride onhydrolysis produce NH3b)Haber’s process of ammonia formation is exothermicc)NH3 dissolves AgCI forming a soluble complexd)Ammonia is a Lewis base as well as Bronsted-Lowry baseCorrect answer is option 'A,B,C,D'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Regarding ammonia correct statements area)A nitride onhydrolysis produce NH3b)Haber’s process of ammonia formation is exothermicc)NH3 dissolves AgCI forming a soluble complexd)Ammonia is a Lewis base as well as Bronsted-Lowry baseCorrect answer is option 'A,B,C,D'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Regarding ammonia correct statements area)A nitride onhydrolysis produce NH3b)Haber’s process of ammonia formation is exothermicc)NH3 dissolves AgCI forming a soluble complexd)Ammonia is a Lewis base as well as Bronsted-Lowry baseCorrect answer is option 'A,B,C,D'. Can you explain this answer?.
Regarding ammonia correct statements area)A nitride onhydrolysis produce NH3b)Haber’s process of ammonia formation is exothermicc)NH3 dissolves AgCI forming a soluble complexd)Ammonia is a Lewis base as well as Bronsted-Lowry baseCorrect answer is option 'A,B,C,D'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Regarding ammonia correct statements area)A nitride onhydrolysis produce NH3b)Haber’s process of ammonia formation is exothermicc)NH3 dissolves AgCI forming a soluble complexd)Ammonia is a Lewis base as well as Bronsted-Lowry baseCorrect answer is option 'A,B,C,D'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Regarding ammonia correct statements area)A nitride onhydrolysis produce NH3b)Haber’s process of ammonia formation is exothermicc)NH3 dissolves AgCI forming a soluble complexd)Ammonia is a Lewis base as well as Bronsted-Lowry baseCorrect answer is option 'A,B,C,D'. Can you explain this answer?.
Solutions for Regarding ammonia correct statements area)A nitride onhydrolysis produce NH3b)Haber’s process of ammonia formation is exothermicc)NH3 dissolves AgCI forming a soluble complexd)Ammonia is a Lewis base as well as Bronsted-Lowry baseCorrect answer is option 'A,B,C,D'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Regarding ammonia correct statements area)A nitride onhydrolysis produce NH3b)Haber’s process of ammonia formation is exothermicc)NH3 dissolves AgCI forming a soluble complexd)Ammonia is a Lewis base as well as Bronsted-Lowry baseCorrect answer is option 'A,B,C,D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Regarding ammonia correct statements area)A nitride onhydrolysis produce NH3b)Haber’s process of ammonia formation is exothermicc)NH3 dissolves AgCI forming a soluble complexd)Ammonia is a Lewis base as well as Bronsted-Lowry baseCorrect answer is option 'A,B,C,D'. Can you explain this answer?, a detailed solution for Regarding ammonia correct statements area)A nitride onhydrolysis produce NH3b)Haber’s process of ammonia formation is exothermicc)NH3 dissolves AgCI forming a soluble complexd)Ammonia is a Lewis base as well as Bronsted-Lowry baseCorrect answer is option 'A,B,C,D'. Can you explain this answer? has been provided alongside types of Regarding ammonia correct statements area)A nitride onhydrolysis produce NH3b)Haber’s process of ammonia formation is exothermicc)NH3 dissolves AgCI forming a soluble complexd)Ammonia is a Lewis base as well as Bronsted-Lowry baseCorrect answer is option 'A,B,C,D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Regarding ammonia correct statements area)A nitride onhydrolysis produce NH3b)Haber’s process of ammonia formation is exothermicc)NH3 dissolves AgCI forming a soluble complexd)Ammonia is a Lewis base as well as Bronsted-Lowry baseCorrect answer is option 'A,B,C,D'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.





















