Class 12 Exam > Class 12 Questions > How can be prepare phenol by benzene sulphoni...
Start Learning for Free
How can be prepare phenol by benzene sulphonic acid with mechanism
Most Upvoted Answer
How can be prepare phenol by benzene sulphonic acid with mechanism
Community Answer
How can be prepare phenol by benzene sulphonic acid with mechanism
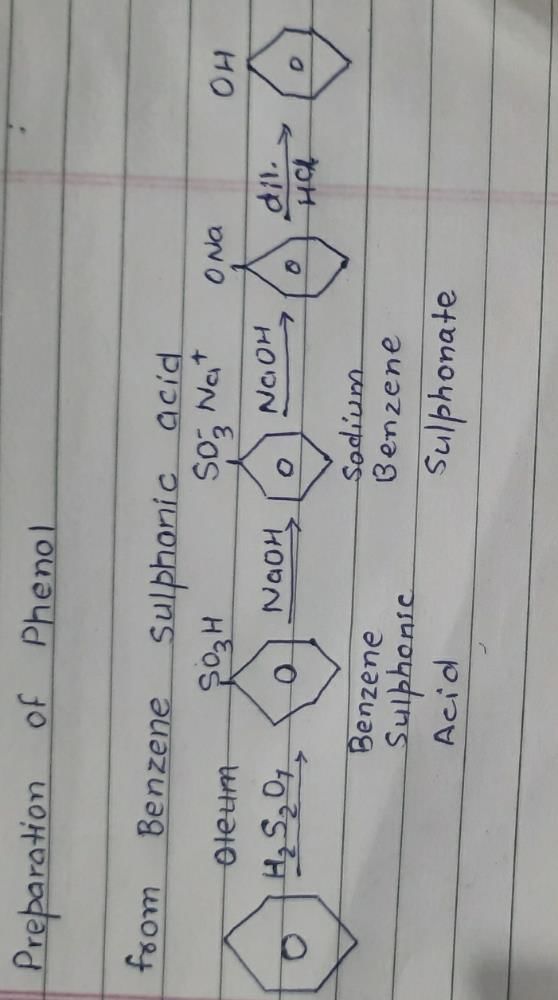
Preparation of Phenol from Benzene Sulphonic Acid
Phenol can be prepared from benzene sulphonic acid through a series of chemical reactions. The main steps involved in this process are as follows:
1. Sulphonation of Benzene:
The first step is the sulphonation of benzene to form benzene sulphonic acid. This reaction is carried out by treating benzene with concentrated sulphuric acid (H2SO4) at a temperature of around 50-60°C. The reaction is as follows:

The electrophilic substitution of benzene occurs in the presence of a strong acid catalyst (H2SO4). The sulphonic acid group (-SO3H) is introduced into the benzene ring, resulting in the formation of benzene sulphonic acid.
2. Conversion of Benzene Sulphonic Acid to Sodium Phenoxide:
The next step involves the conversion of benzene sulphonic acid to sodium phenoxide. This is achieved by treating benzene sulphonic acid with sodium hydroxide (NaOH) or sodium carbonate (Na2CO3) at a high temperature of about 300-350°C. The reaction is as follows:

The sulphonic acid group is replaced by a hydroxyl group (-OH), resulting in the formation of sodium phenoxide.
3. Acidification of Sodium Phenoxide to Obtain Phenol:
The final step involves the acidification of sodium phenoxide to obtain phenol. This is done by treating the sodium phenoxide solution with dilute hydrochloric acid (HCl). The reaction is as follows:

The hydroxide group (-OH) of sodium phenoxide is protonated by the hydrogen ion (H+) from the hydrochloric acid, resulting in the formation of phenol. Sodium chloride (NaCl) is formed as a byproduct in this reaction.
The obtained phenol can be further purified by processes like distillation or recrystallization.
In summary, phenol can be prepared from benzene sulphonic acid by sulphonation of benzene to form benzene sulphonic acid, conversion of benzene sulphonic acid to sodium phenoxide, and acidification of sodium phenoxide to obtain phenol. Each step involves specific reactants and conditions, leading to the formation of phenol as the final product.
Phenol can be prepared from benzene sulphonic acid through a series of chemical reactions. The main steps involved in this process are as follows:
1. Sulphonation of Benzene:
The first step is the sulphonation of benzene to form benzene sulphonic acid. This reaction is carried out by treating benzene with concentrated sulphuric acid (H2SO4) at a temperature of around 50-60°C. The reaction is as follows:

The electrophilic substitution of benzene occurs in the presence of a strong acid catalyst (H2SO4). The sulphonic acid group (-SO3H) is introduced into the benzene ring, resulting in the formation of benzene sulphonic acid.
2. Conversion of Benzene Sulphonic Acid to Sodium Phenoxide:
The next step involves the conversion of benzene sulphonic acid to sodium phenoxide. This is achieved by treating benzene sulphonic acid with sodium hydroxide (NaOH) or sodium carbonate (Na2CO3) at a high temperature of about 300-350°C. The reaction is as follows:

The sulphonic acid group is replaced by a hydroxyl group (-OH), resulting in the formation of sodium phenoxide.
3. Acidification of Sodium Phenoxide to Obtain Phenol:
The final step involves the acidification of sodium phenoxide to obtain phenol. This is done by treating the sodium phenoxide solution with dilute hydrochloric acid (HCl). The reaction is as follows:

The hydroxide group (-OH) of sodium phenoxide is protonated by the hydrogen ion (H+) from the hydrochloric acid, resulting in the formation of phenol. Sodium chloride (NaCl) is formed as a byproduct in this reaction.
The obtained phenol can be further purified by processes like distillation or recrystallization.
In summary, phenol can be prepared from benzene sulphonic acid by sulphonation of benzene to form benzene sulphonic acid, conversion of benzene sulphonic acid to sodium phenoxide, and acidification of sodium phenoxide to obtain phenol. Each step involves specific reactants and conditions, leading to the formation of phenol as the final product.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
How can be prepare phenol by benzene sulphonic acid with mechanism
Question Description
How can be prepare phenol by benzene sulphonic acid with mechanism for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about How can be prepare phenol by benzene sulphonic acid with mechanism covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for How can be prepare phenol by benzene sulphonic acid with mechanism.
How can be prepare phenol by benzene sulphonic acid with mechanism for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about How can be prepare phenol by benzene sulphonic acid with mechanism covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for How can be prepare phenol by benzene sulphonic acid with mechanism.
Solutions for How can be prepare phenol by benzene sulphonic acid with mechanism in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of How can be prepare phenol by benzene sulphonic acid with mechanism defined & explained in the simplest way possible. Besides giving the explanation of
How can be prepare phenol by benzene sulphonic acid with mechanism, a detailed solution for How can be prepare phenol by benzene sulphonic acid with mechanism has been provided alongside types of How can be prepare phenol by benzene sulphonic acid with mechanism theory, EduRev gives you an
ample number of questions to practice How can be prepare phenol by benzene sulphonic acid with mechanism tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.



















