Physics Exam > Physics Questions > For a particle in a 3 dimensional boxa)The en...
Start Learning for Free
For a particle in a 3 dimensional box
- a)The energy levels are evenly spaced
- b)Ground state energy is non zero
- c)The second excited state is 3 fold degenerate
- d)The second excited state is non degenerate
Correct answer is option 'B,C'. Can you explain this answer?
Verified Answer
For a particle in a 3 dimensional boxa)The energy levels are evenly sp...
Second excited state
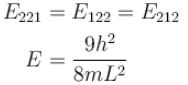
3 fold degenerate!
The correct answers are: Ground state energy is non zero, The second excited state is 3 fold degenerate
Most Upvoted Answer
For a particle in a 3 dimensional boxa)The energy levels are evenly sp...
Particle in a 3-dimensional box:
The particle in a 3-dimensional box is a commonly used model in quantum mechanics to study the behavior of a particle confined within a finite region. In this model, the particle is assumed to be free to move in three dimensions, but is restricted to the boundaries of the box.
a) The energy levels are evenly spaced:
In a 3-dimensional box, the energy levels of the particle are not evenly spaced. In fact, the energy levels follow a pattern where the energy increases as the quantum numbers associated with each level increase. The energy eigenvalues of a particle in a box can be determined by solving the Schrödinger equation for the system.
b) Ground state energy is non-zero:
The ground state of a particle in a 3-dimensional box refers to the lowest energy level that the particle can occupy. In this state, all three quantum numbers associated with the particle's motion are at their lowest possible values. The ground state energy of a particle in a box is indeed non-zero and it corresponds to the minimum energy that the particle can have while still being confined within the box.
c) The second excited state is 3-fold degenerate:
Degeneracy refers to the phenomenon where multiple states have the same energy. In the case of a particle in a 3-dimensional box, the energy levels can be classified according to the number of nodes along each axis. The second excited state corresponds to having two nodes along one axis and one node along each of the other two axes. This state is indeed 3-fold degenerate because there are three different combinations of quantum numbers that can yield the same energy level.
d) The second excited state is non-degenerate:
Contrary to option d, the second excited state of a particle in a 3-dimensional box is degenerate. As mentioned earlier, this state corresponds to having two nodes along one axis and one node along each of the other two axes. The degeneracy arises from the fact that there are multiple combinations of quantum numbers that satisfy this condition and yield the same energy level.
Therefore, the correct answers for this question are option b) - the ground state energy is non-zero, and option c) - the second excited state is 3-fold degenerate.
The particle in a 3-dimensional box is a commonly used model in quantum mechanics to study the behavior of a particle confined within a finite region. In this model, the particle is assumed to be free to move in three dimensions, but is restricted to the boundaries of the box.
a) The energy levels are evenly spaced:
In a 3-dimensional box, the energy levels of the particle are not evenly spaced. In fact, the energy levels follow a pattern where the energy increases as the quantum numbers associated with each level increase. The energy eigenvalues of a particle in a box can be determined by solving the Schrödinger equation for the system.
b) Ground state energy is non-zero:
The ground state of a particle in a 3-dimensional box refers to the lowest energy level that the particle can occupy. In this state, all three quantum numbers associated with the particle's motion are at their lowest possible values. The ground state energy of a particle in a box is indeed non-zero and it corresponds to the minimum energy that the particle can have while still being confined within the box.
c) The second excited state is 3-fold degenerate:
Degeneracy refers to the phenomenon where multiple states have the same energy. In the case of a particle in a 3-dimensional box, the energy levels can be classified according to the number of nodes along each axis. The second excited state corresponds to having two nodes along one axis and one node along each of the other two axes. This state is indeed 3-fold degenerate because there are three different combinations of quantum numbers that can yield the same energy level.
d) The second excited state is non-degenerate:
Contrary to option d, the second excited state of a particle in a 3-dimensional box is degenerate. As mentioned earlier, this state corresponds to having two nodes along one axis and one node along each of the other two axes. The degeneracy arises from the fact that there are multiple combinations of quantum numbers that satisfy this condition and yield the same energy level.
Therefore, the correct answers for this question are option b) - the ground state energy is non-zero, and option c) - the second excited state is 3-fold degenerate.

|
Explore Courses for Physics exam
|

|
Question Description
For a particle in a 3 dimensional boxa)The energy levels are evenly spacedb)Ground state energy is non zeroc)The second excited state is 3 fold degenerated)The second excited state is non degenerateCorrect answer is option 'B,C'. Can you explain this answer? for Physics 2025 is part of Physics preparation. The Question and answers have been prepared according to the Physics exam syllabus. Information about For a particle in a 3 dimensional boxa)The energy levels are evenly spacedb)Ground state energy is non zeroc)The second excited state is 3 fold degenerated)The second excited state is non degenerateCorrect answer is option 'B,C'. Can you explain this answer? covers all topics & solutions for Physics 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for For a particle in a 3 dimensional boxa)The energy levels are evenly spacedb)Ground state energy is non zeroc)The second excited state is 3 fold degenerated)The second excited state is non degenerateCorrect answer is option 'B,C'. Can you explain this answer?.
For a particle in a 3 dimensional boxa)The energy levels are evenly spacedb)Ground state energy is non zeroc)The second excited state is 3 fold degenerated)The second excited state is non degenerateCorrect answer is option 'B,C'. Can you explain this answer? for Physics 2025 is part of Physics preparation. The Question and answers have been prepared according to the Physics exam syllabus. Information about For a particle in a 3 dimensional boxa)The energy levels are evenly spacedb)Ground state energy is non zeroc)The second excited state is 3 fold degenerated)The second excited state is non degenerateCorrect answer is option 'B,C'. Can you explain this answer? covers all topics & solutions for Physics 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for For a particle in a 3 dimensional boxa)The energy levels are evenly spacedb)Ground state energy is non zeroc)The second excited state is 3 fold degenerated)The second excited state is non degenerateCorrect answer is option 'B,C'. Can you explain this answer?.
Solutions for For a particle in a 3 dimensional boxa)The energy levels are evenly spacedb)Ground state energy is non zeroc)The second excited state is 3 fold degenerated)The second excited state is non degenerateCorrect answer is option 'B,C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Physics.
Download more important topics, notes, lectures and mock test series for Physics Exam by signing up for free.
Here you can find the meaning of For a particle in a 3 dimensional boxa)The energy levels are evenly spacedb)Ground state energy is non zeroc)The second excited state is 3 fold degenerated)The second excited state is non degenerateCorrect answer is option 'B,C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
For a particle in a 3 dimensional boxa)The energy levels are evenly spacedb)Ground state energy is non zeroc)The second excited state is 3 fold degenerated)The second excited state is non degenerateCorrect answer is option 'B,C'. Can you explain this answer?, a detailed solution for For a particle in a 3 dimensional boxa)The energy levels are evenly spacedb)Ground state energy is non zeroc)The second excited state is 3 fold degenerated)The second excited state is non degenerateCorrect answer is option 'B,C'. Can you explain this answer? has been provided alongside types of For a particle in a 3 dimensional boxa)The energy levels are evenly spacedb)Ground state energy is non zeroc)The second excited state is 3 fold degenerated)The second excited state is non degenerateCorrect answer is option 'B,C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice For a particle in a 3 dimensional boxa)The energy levels are evenly spacedb)Ground state energy is non zeroc)The second excited state is 3 fold degenerated)The second excited state is non degenerateCorrect answer is option 'B,C'. Can you explain this answer? tests, examples and also practice Physics tests.

|
Explore Courses for Physics exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























