JEE Exam > JEE Questions > Direction: Question is assertion reason type....
Start Learning for Free
Direction: Question is assertion reason type. These question contains two statements Statement I (Assertion), Statement II (Reason). These question also has four alternative choices, only one of which is correct. You have to select the correct choices from the codes (a), (b), (c) and (d) given below:
Statement I : Detection of chlorine in 2, 4, 6 -trinitrochlorrobenzene can be done directly by addition aq. AgNo3 solution.
Statement II: C-Cl bond is weakened by electron withdrawing - NO2 group
- a)Statement I is true; Statement II is true; Statement II is not a correct explanation for Statement I.
- b)Statement I is true; Statement II is false.
- c)Statement I is false; Statement II is true.
- d)Statement I is true; Statement II is true; Statement II is not a correct explanation for Statement I.
Correct answer is option 'D'. Can you explain this answer?
Verified Answer
Direction: Question is assertion reason type. These question contains ...
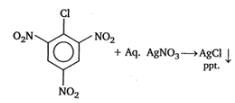
2,4, 6 - trinitrocholorobenzene The presence of electron withdrawing group (like -NO2) makes the necleophilic aroatic substitution easier, as it decrease the setrength of C-Cl bond. thus -Cl give ppt. of AgCl with aq. AgNo3
 This question is part of UPSC exam. View all JEE courses
This question is part of UPSC exam. View all JEE courses
Most Upvoted Answer
Direction: Question is assertion reason type. These question contains ...
Explanation of the Assertion and Reason
The question revolves around the detection of chlorine in 2, 4, 6-trinitrochlorobenzene and the influence of nitro groups on the C-Cl bond.
Statement I: Detection of Chlorine
- The statement is true.
- Chlorine can be detected in 2, 4, 6-trinitrochlorobenzene by adding aqueous AgNO3 solution.
- This method relies on the formation of a precipitate (AgCl) when chlorine is present.
Statement II: Influence of Nitro Groups
- This statement is also true.
- The C-Cl bond in 2, 4, 6-trinitrochlorobenzene is weakened due to the presence of electron-withdrawing nitro (-NO2) groups.
- The nitro groups stabilize the positive charge in the molecule, making the C-Cl bond more susceptible to nucleophilic attack.
Relationship Between Statement I and Statement II
- Although both statements are true, Statement II does not directly explain Statement I.
- The detection of chlorine via AgNO3 does not depend solely on the weakening of the C-Cl bond; it is primarily a result of the precipitation reaction with silver ions.
Conclusion
- Therefore, the correct choice is option D:
- Statement I is true; Statement II is true; Statement II is not a correct explanation for Statement I.
This ensures a clear understanding of the relationship between the assertion and the reason provided.
The question revolves around the detection of chlorine in 2, 4, 6-trinitrochlorobenzene and the influence of nitro groups on the C-Cl bond.
Statement I: Detection of Chlorine
- The statement is true.
- Chlorine can be detected in 2, 4, 6-trinitrochlorobenzene by adding aqueous AgNO3 solution.
- This method relies on the formation of a precipitate (AgCl) when chlorine is present.
Statement II: Influence of Nitro Groups
- This statement is also true.
- The C-Cl bond in 2, 4, 6-trinitrochlorobenzene is weakened due to the presence of electron-withdrawing nitro (-NO2) groups.
- The nitro groups stabilize the positive charge in the molecule, making the C-Cl bond more susceptible to nucleophilic attack.
Relationship Between Statement I and Statement II
- Although both statements are true, Statement II does not directly explain Statement I.
- The detection of chlorine via AgNO3 does not depend solely on the weakening of the C-Cl bond; it is primarily a result of the precipitation reaction with silver ions.
Conclusion
- Therefore, the correct choice is option D:
- Statement I is true; Statement II is true; Statement II is not a correct explanation for Statement I.
This ensures a clear understanding of the relationship between the assertion and the reason provided.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
Question Description
Direction: Question is assertion reason type. These question contains two statements Statement I (Assertion), Statement II (Reason). These question also has four alternative choices, only one of which is correct. You have to select the correct choices from the codes (a), (b), (c) and (d) given below:Statement I : Detection of chlorine in 2, 4, 6 -trinitrochlorrobenzene can be done directly by addition aq. AgNo3solution.Statement II: C-Cl bond is weakened by electron withdrawing - NO2groupa)Statement I is true; Statement II is true; Statement II is not a correct explanation for Statement I.b)Statement I is true; Statement II is false.c)Statement I is false; Statement II is true.d)Statement I is true; Statement II is true; Statement II is not a correct explanation for Statement I.Correct answer is option 'D'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Direction: Question is assertion reason type. These question contains two statements Statement I (Assertion), Statement II (Reason). These question also has four alternative choices, only one of which is correct. You have to select the correct choices from the codes (a), (b), (c) and (d) given below:Statement I : Detection of chlorine in 2, 4, 6 -trinitrochlorrobenzene can be done directly by addition aq. AgNo3solution.Statement II: C-Cl bond is weakened by electron withdrawing - NO2groupa)Statement I is true; Statement II is true; Statement II is not a correct explanation for Statement I.b)Statement I is true; Statement II is false.c)Statement I is false; Statement II is true.d)Statement I is true; Statement II is true; Statement II is not a correct explanation for Statement I.Correct answer is option 'D'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Direction: Question is assertion reason type. These question contains two statements Statement I (Assertion), Statement II (Reason). These question also has four alternative choices, only one of which is correct. You have to select the correct choices from the codes (a), (b), (c) and (d) given below:Statement I : Detection of chlorine in 2, 4, 6 -trinitrochlorrobenzene can be done directly by addition aq. AgNo3solution.Statement II: C-Cl bond is weakened by electron withdrawing - NO2groupa)Statement I is true; Statement II is true; Statement II is not a correct explanation for Statement I.b)Statement I is true; Statement II is false.c)Statement I is false; Statement II is true.d)Statement I is true; Statement II is true; Statement II is not a correct explanation for Statement I.Correct answer is option 'D'. Can you explain this answer?.
Direction: Question is assertion reason type. These question contains two statements Statement I (Assertion), Statement II (Reason). These question also has four alternative choices, only one of which is correct. You have to select the correct choices from the codes (a), (b), (c) and (d) given below:Statement I : Detection of chlorine in 2, 4, 6 -trinitrochlorrobenzene can be done directly by addition aq. AgNo3solution.Statement II: C-Cl bond is weakened by electron withdrawing - NO2groupa)Statement I is true; Statement II is true; Statement II is not a correct explanation for Statement I.b)Statement I is true; Statement II is false.c)Statement I is false; Statement II is true.d)Statement I is true; Statement II is true; Statement II is not a correct explanation for Statement I.Correct answer is option 'D'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Direction: Question is assertion reason type. These question contains two statements Statement I (Assertion), Statement II (Reason). These question also has four alternative choices, only one of which is correct. You have to select the correct choices from the codes (a), (b), (c) and (d) given below:Statement I : Detection of chlorine in 2, 4, 6 -trinitrochlorrobenzene can be done directly by addition aq. AgNo3solution.Statement II: C-Cl bond is weakened by electron withdrawing - NO2groupa)Statement I is true; Statement II is true; Statement II is not a correct explanation for Statement I.b)Statement I is true; Statement II is false.c)Statement I is false; Statement II is true.d)Statement I is true; Statement II is true; Statement II is not a correct explanation for Statement I.Correct answer is option 'D'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Direction: Question is assertion reason type. These question contains two statements Statement I (Assertion), Statement II (Reason). These question also has four alternative choices, only one of which is correct. You have to select the correct choices from the codes (a), (b), (c) and (d) given below:Statement I : Detection of chlorine in 2, 4, 6 -trinitrochlorrobenzene can be done directly by addition aq. AgNo3solution.Statement II: C-Cl bond is weakened by electron withdrawing - NO2groupa)Statement I is true; Statement II is true; Statement II is not a correct explanation for Statement I.b)Statement I is true; Statement II is false.c)Statement I is false; Statement II is true.d)Statement I is true; Statement II is true; Statement II is not a correct explanation for Statement I.Correct answer is option 'D'. Can you explain this answer?.
Solutions for Direction: Question is assertion reason type. These question contains two statements Statement I (Assertion), Statement II (Reason). These question also has four alternative choices, only one of which is correct. You have to select the correct choices from the codes (a), (b), (c) and (d) given below:Statement I : Detection of chlorine in 2, 4, 6 -trinitrochlorrobenzene can be done directly by addition aq. AgNo3solution.Statement II: C-Cl bond is weakened by electron withdrawing - NO2groupa)Statement I is true; Statement II is true; Statement II is not a correct explanation for Statement I.b)Statement I is true; Statement II is false.c)Statement I is false; Statement II is true.d)Statement I is true; Statement II is true; Statement II is not a correct explanation for Statement I.Correct answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Direction: Question is assertion reason type. These question contains two statements Statement I (Assertion), Statement II (Reason). These question also has four alternative choices, only one of which is correct. You have to select the correct choices from the codes (a), (b), (c) and (d) given below:Statement I : Detection of chlorine in 2, 4, 6 -trinitrochlorrobenzene can be done directly by addition aq. AgNo3solution.Statement II: C-Cl bond is weakened by electron withdrawing - NO2groupa)Statement I is true; Statement II is true; Statement II is not a correct explanation for Statement I.b)Statement I is true; Statement II is false.c)Statement I is false; Statement II is true.d)Statement I is true; Statement II is true; Statement II is not a correct explanation for Statement I.Correct answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Direction: Question is assertion reason type. These question contains two statements Statement I (Assertion), Statement II (Reason). These question also has four alternative choices, only one of which is correct. You have to select the correct choices from the codes (a), (b), (c) and (d) given below:Statement I : Detection of chlorine in 2, 4, 6 -trinitrochlorrobenzene can be done directly by addition aq. AgNo3solution.Statement II: C-Cl bond is weakened by electron withdrawing - NO2groupa)Statement I is true; Statement II is true; Statement II is not a correct explanation for Statement I.b)Statement I is true; Statement II is false.c)Statement I is false; Statement II is true.d)Statement I is true; Statement II is true; Statement II is not a correct explanation for Statement I.Correct answer is option 'D'. Can you explain this answer?, a detailed solution for Direction: Question is assertion reason type. These question contains two statements Statement I (Assertion), Statement II (Reason). These question also has four alternative choices, only one of which is correct. You have to select the correct choices from the codes (a), (b), (c) and (d) given below:Statement I : Detection of chlorine in 2, 4, 6 -trinitrochlorrobenzene can be done directly by addition aq. AgNo3solution.Statement II: C-Cl bond is weakened by electron withdrawing - NO2groupa)Statement I is true; Statement II is true; Statement II is not a correct explanation for Statement I.b)Statement I is true; Statement II is false.c)Statement I is false; Statement II is true.d)Statement I is true; Statement II is true; Statement II is not a correct explanation for Statement I.Correct answer is option 'D'. Can you explain this answer? has been provided alongside types of Direction: Question is assertion reason type. These question contains two statements Statement I (Assertion), Statement II (Reason). These question also has four alternative choices, only one of which is correct. You have to select the correct choices from the codes (a), (b), (c) and (d) given below:Statement I : Detection of chlorine in 2, 4, 6 -trinitrochlorrobenzene can be done directly by addition aq. AgNo3solution.Statement II: C-Cl bond is weakened by electron withdrawing - NO2groupa)Statement I is true; Statement II is true; Statement II is not a correct explanation for Statement I.b)Statement I is true; Statement II is false.c)Statement I is false; Statement II is true.d)Statement I is true; Statement II is true; Statement II is not a correct explanation for Statement I.Correct answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Direction: Question is assertion reason type. These question contains two statements Statement I (Assertion), Statement II (Reason). These question also has four alternative choices, only one of which is correct. You have to select the correct choices from the codes (a), (b), (c) and (d) given below:Statement I : Detection of chlorine in 2, 4, 6 -trinitrochlorrobenzene can be done directly by addition aq. AgNo3solution.Statement II: C-Cl bond is weakened by electron withdrawing - NO2groupa)Statement I is true; Statement II is true; Statement II is not a correct explanation for Statement I.b)Statement I is true; Statement II is false.c)Statement I is false; Statement II is true.d)Statement I is true; Statement II is true; Statement II is not a correct explanation for Statement I.Correct answer is option 'D'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup to solve all Doubts
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.





















