Chemistry Exam > Chemistry Questions > On nitration of quinoline, Nitration occur wh...
Start Learning for Free
On nitration of quinoline, Nitration occur which position at quinoline as a major product
Correct answer is '5'. Can you explain this answer?
Verified Answer
On nitration of quinoline, Nitration occur which position at quinoline...
Most Upvoted Answer
On nitration of quinoline, Nitration occur which position at quinoline...
Nitration of Quinoline
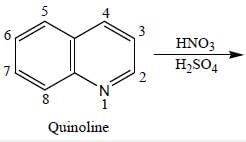
The nitration of quinoline involves the substitution of a nitro group (-NO2) onto the quinoline molecule. Nitration is a common reaction in organic chemistry and is often used to introduce functional groups onto aromatic compounds. In the case of quinoline, the major product of nitration is formed by the substitution of the nitro group at the 5-position of the quinoline ring.
Explanation:
1. Structure of Quinoline
Quinoline is a heterocyclic aromatic compound consisting of a benzene ring fused to a pyridine ring. It has a nitrogen atom in the 1-position and a double bond between the 2- and 3-carbon atoms. The numbering of the carbon atoms in the quinoline ring starts from the nitrogen atom.
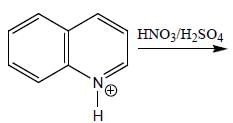
2. Nitration Reaction
During the nitration reaction, a mixture of nitric acid (HNO3) and sulfuric acid (H2SO4) is used as the nitrating agent. The sulfuric acid serves as a catalyst and also helps in the formation of the nitronium ion (NO2+), which is the electrophile responsible for the substitution reaction.
3. Electrophilic Aromatic Substitution
The nitronium ion (NO2+) acts as an electrophile and attacks the electron-rich aromatic ring of quinoline. The reaction proceeds through an electrophilic aromatic substitution mechanism. The nitrogen atom in the quinoline ring can donate electron density to the ring, making the carbon atoms in the 5-position more nucleophilic compared to the other positions.
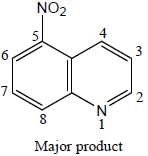
4. Attack at the 5-Position
Due to the greater nucleophilicity of the 5-position carbon atom, the nitronium ion preferentially attacks this position. The electrophilic attack leads to the substitution of a nitro group (-NO2) at the 5-position of the quinoline ring. This substitution reaction is regioselective, meaning that it occurs selectively at a specific position.
5. Major Product Formation
As a result of the preferential attack at the 5-position, the major product of the nitration of quinoline is formed by the substitution of the nitro group at this position. The other positions on the quinoline ring are less nucleophilic and hence less reactive towards electrophilic attack.
Overall, the nitration of quinoline produces the major product with a nitro group substituted at the 5-position of the quinoline ring. This regioselectivity can be attributed to the electronic effects of the nitrogen atom in the quinoline ring, which enhances the nucleophilicity of the 5-position carbon atom.
The nitration of quinoline involves the substitution of a nitro group (-NO2) onto the quinoline molecule. Nitration is a common reaction in organic chemistry and is often used to introduce functional groups onto aromatic compounds. In the case of quinoline, the major product of nitration is formed by the substitution of the nitro group at the 5-position of the quinoline ring.
Explanation:
1. Structure of Quinoline
Quinoline is a heterocyclic aromatic compound consisting of a benzene ring fused to a pyridine ring. It has a nitrogen atom in the 1-position and a double bond between the 2- and 3-carbon atoms. The numbering of the carbon atoms in the quinoline ring starts from the nitrogen atom.
2. Nitration Reaction
During the nitration reaction, a mixture of nitric acid (HNO3) and sulfuric acid (H2SO4) is used as the nitrating agent. The sulfuric acid serves as a catalyst and also helps in the formation of the nitronium ion (NO2+), which is the electrophile responsible for the substitution reaction.
3. Electrophilic Aromatic Substitution
The nitronium ion (NO2+) acts as an electrophile and attacks the electron-rich aromatic ring of quinoline. The reaction proceeds through an electrophilic aromatic substitution mechanism. The nitrogen atom in the quinoline ring can donate electron density to the ring, making the carbon atoms in the 5-position more nucleophilic compared to the other positions.
4. Attack at the 5-Position
Due to the greater nucleophilicity of the 5-position carbon atom, the nitronium ion preferentially attacks this position. The electrophilic attack leads to the substitution of a nitro group (-NO2) at the 5-position of the quinoline ring. This substitution reaction is regioselective, meaning that it occurs selectively at a specific position.
5. Major Product Formation
As a result of the preferential attack at the 5-position, the major product of the nitration of quinoline is formed by the substitution of the nitro group at this position. The other positions on the quinoline ring are less nucleophilic and hence less reactive towards electrophilic attack.
Overall, the nitration of quinoline produces the major product with a nitro group substituted at the 5-position of the quinoline ring. This regioselectivity can be attributed to the electronic effects of the nitrogen atom in the quinoline ring, which enhances the nucleophilicity of the 5-position carbon atom.

|
Explore Courses for Chemistry exam
|

|
Similar Chemistry Doubts
On nitration of quinoline, Nitration occur which position at quinoline as a major productCorrect answer is '5'. Can you explain this answer?
Question Description
On nitration of quinoline, Nitration occur which position at quinoline as a major productCorrect answer is '5'. Can you explain this answer? for Chemistry 2025 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about On nitration of quinoline, Nitration occur which position at quinoline as a major productCorrect answer is '5'. Can you explain this answer? covers all topics & solutions for Chemistry 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for On nitration of quinoline, Nitration occur which position at quinoline as a major productCorrect answer is '5'. Can you explain this answer?.
On nitration of quinoline, Nitration occur which position at quinoline as a major productCorrect answer is '5'. Can you explain this answer? for Chemistry 2025 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about On nitration of quinoline, Nitration occur which position at quinoline as a major productCorrect answer is '5'. Can you explain this answer? covers all topics & solutions for Chemistry 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for On nitration of quinoline, Nitration occur which position at quinoline as a major productCorrect answer is '5'. Can you explain this answer?.
Solutions for On nitration of quinoline, Nitration occur which position at quinoline as a major productCorrect answer is '5'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of On nitration of quinoline, Nitration occur which position at quinoline as a major productCorrect answer is '5'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
On nitration of quinoline, Nitration occur which position at quinoline as a major productCorrect answer is '5'. Can you explain this answer?, a detailed solution for On nitration of quinoline, Nitration occur which position at quinoline as a major productCorrect answer is '5'. Can you explain this answer? has been provided alongside types of On nitration of quinoline, Nitration occur which position at quinoline as a major productCorrect answer is '5'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice On nitration of quinoline, Nitration occur which position at quinoline as a major productCorrect answer is '5'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Signup to solve all Doubts
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.





















