Chemistry Exam > Chemistry Questions > The total number of line in M-B spectrum of F...
Start Learning for Free
The total number of line in M-B spectrum of Fe(CO)5 is ________
Correct answer is '6'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
The total number of line in M-B spectrum of Fe(CO)5is ________Correct ...
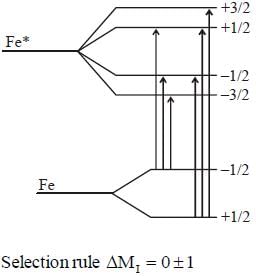
Most Upvoted Answer
The total number of line in M-B spectrum of Fe(CO)5is ________Correct ...
Introduction:
The M-B spectrum refers to the Molecule-Beam spectrum, which is a technique used to study the electronic structure and bonding in molecules. In this technique, a molecular beam is passed through a magnetic field, causing the molecule to undergo quantized rotational and vibrational transitions. These transitions result in the absorption or emission of radiation, which can be observed as spectral lines.
Fe(CO)5:
Fe(CO)5 is the chemical formula for iron pentacarbonyl. It is a coordination complex consisting of an iron atom bonded to five carbon monoxide (CO) ligands. The molecule has a trigonal bipyramidal geometry, with the iron atom at the center and the CO ligands arranged symmetrically around it.
Line in M-B Spectrum:
The number of lines observed in the M-B spectrum of a molecule depends on the number of possible transitions between different energy levels. In the case of Fe(CO)5, the number of lines can be determined by considering the possible rotational and vibrational transitions.
Rotational Transitions:
Rotational transitions occur when a molecule changes its rotational energy level. Fe(CO)5 has a trigonal bipyramidal geometry, which allows for three different rotational axes: two perpendicular to the plane of the CO ligands (a and b axes) and one along the axis of the molecule (c axis). Each rotational axis can have different energy levels, resulting in rotational transitions.
In the M-B spectrum, rotational transitions are observed as spectral lines. For a molecule with a trigonal bipyramidal geometry, there are a total of six possible rotational transitions, corresponding to the three rotational axes (a, b, and c) and two possible energy levels for each axis. Therefore, the total number of lines due to rotational transitions in the M-B spectrum of Fe(CO)5 is six.
Vibrational Transitions:
Vibrational transitions occur when a molecule changes its vibrational energy level. Fe(CO)5 has multiple vibrational modes, including symmetric stretching, asymmetric stretching, and bending modes. Each vibrational mode can have different energy levels, resulting in vibrational transitions.
However, the question specifically asks for the total number of lines in the M-B spectrum, not just the vibrational transitions. Therefore, we only consider the rotational transitions for this calculation.
Conclusion:
In conclusion, the total number of lines in the M-B spectrum of Fe(CO)5 is six. This is due to the three rotational axes (a, b, and c) and two possible energy levels for each axis, resulting in six possible rotational transitions.
The M-B spectrum refers to the Molecule-Beam spectrum, which is a technique used to study the electronic structure and bonding in molecules. In this technique, a molecular beam is passed through a magnetic field, causing the molecule to undergo quantized rotational and vibrational transitions. These transitions result in the absorption or emission of radiation, which can be observed as spectral lines.
Fe(CO)5:
Fe(CO)5 is the chemical formula for iron pentacarbonyl. It is a coordination complex consisting of an iron atom bonded to five carbon monoxide (CO) ligands. The molecule has a trigonal bipyramidal geometry, with the iron atom at the center and the CO ligands arranged symmetrically around it.
Line in M-B Spectrum:
The number of lines observed in the M-B spectrum of a molecule depends on the number of possible transitions between different energy levels. In the case of Fe(CO)5, the number of lines can be determined by considering the possible rotational and vibrational transitions.
Rotational Transitions:
Rotational transitions occur when a molecule changes its rotational energy level. Fe(CO)5 has a trigonal bipyramidal geometry, which allows for three different rotational axes: two perpendicular to the plane of the CO ligands (a and b axes) and one along the axis of the molecule (c axis). Each rotational axis can have different energy levels, resulting in rotational transitions.
In the M-B spectrum, rotational transitions are observed as spectral lines. For a molecule with a trigonal bipyramidal geometry, there are a total of six possible rotational transitions, corresponding to the three rotational axes (a, b, and c) and two possible energy levels for each axis. Therefore, the total number of lines due to rotational transitions in the M-B spectrum of Fe(CO)5 is six.
Vibrational Transitions:
Vibrational transitions occur when a molecule changes its vibrational energy level. Fe(CO)5 has multiple vibrational modes, including symmetric stretching, asymmetric stretching, and bending modes. Each vibrational mode can have different energy levels, resulting in vibrational transitions.
However, the question specifically asks for the total number of lines in the M-B spectrum, not just the vibrational transitions. Therefore, we only consider the rotational transitions for this calculation.
Conclusion:
In conclusion, the total number of lines in the M-B spectrum of Fe(CO)5 is six. This is due to the three rotational axes (a, b, and c) and two possible energy levels for each axis, resulting in six possible rotational transitions.

|
Explore Courses for Chemistry exam
|

|
Similar Chemistry Doubts
The total number of line in M-B spectrum of Fe(CO)5is ________Correct answer is '6'. Can you explain this answer?
Question Description
The total number of line in M-B spectrum of Fe(CO)5is ________Correct answer is '6'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about The total number of line in M-B spectrum of Fe(CO)5is ________Correct answer is '6'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The total number of line in M-B spectrum of Fe(CO)5is ________Correct answer is '6'. Can you explain this answer?.
The total number of line in M-B spectrum of Fe(CO)5is ________Correct answer is '6'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about The total number of line in M-B spectrum of Fe(CO)5is ________Correct answer is '6'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The total number of line in M-B spectrum of Fe(CO)5is ________Correct answer is '6'. Can you explain this answer?.
Solutions for The total number of line in M-B spectrum of Fe(CO)5is ________Correct answer is '6'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of The total number of line in M-B spectrum of Fe(CO)5is ________Correct answer is '6'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The total number of line in M-B spectrum of Fe(CO)5is ________Correct answer is '6'. Can you explain this answer?, a detailed solution for The total number of line in M-B spectrum of Fe(CO)5is ________Correct answer is '6'. Can you explain this answer? has been provided alongside types of The total number of line in M-B spectrum of Fe(CO)5is ________Correct answer is '6'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The total number of line in M-B spectrum of Fe(CO)5is ________Correct answer is '6'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















