JEE Exam > JEE Questions > When nitrobenzene is treated with Br2 in pres...
Start Learning for Free
When nitrobenzene is treated with Br2 in presence of FeBr 3, the major product formed is m-bromonitrobenzene.
Statements which are related to obtain the m-isomer are
Statements which are related to obtain the m-isomer are
- a)The electron density on meta carbon is more than that on ortho and para positions
- b)The intermediate carbonium ion formed after initial attack of Br+ at the meta position is least destabilised
- c)Loss of aromaticity when Br+ attacks at the ortho and para positions and not at meta position
- d)Easier loss of H+ to regain aromaticity from the meta position than from ortho and para positions.
Correct answer is option 'A,D'. Can you explain this answer?
Verified Answer
When nitrobenzene is treated with Br2 in presence of FeBr 3, the major...
TIPS/Formulae : Nitro group decreases the electron density at the meta-position in comparison to ortho and para position due to –I and –M effects.
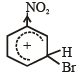
The above intermediate is a resonance hybrid of three structures, hence is more stable than the corresponding intermediate from ortho and paraattack
Most Upvoted Answer
When nitrobenzene is treated with Br2 in presence of FeBr 3, the major...
Explanation:
Statement a: The electron density on the meta carbon is more than that on the ortho and para positions.
- In the presence of FeBr3, the bromination of nitrobenzene occurs via electrophilic aromatic substitution.
- FeBr3 acts as a Lewis acid and forms a complex with Br2, which enhances the electrophilicity of Br2.
- The nitro group (-NO2) is a strong electron-withdrawing group, which decreases the electron density on the aromatic ring.
- As a result, the meta position, which is further away from the nitro group, has a higher electron density compared to the ortho and para positions.
- Therefore, the electrophilic bromine (Br+) attacks the meta position more readily due to the higher electron density.
Statement b: The intermediate carbonium ion formed after the initial attack of Br at the meta position is least destabilized.
- When Br+ attacks the meta position, it forms a resonance-stabilized intermediate carbonium ion.
- The resonance structures of the intermediate carbonium ion show that the positive charge is delocalized over the meta carbon and the adjacent carbon atoms.
- This delocalization of positive charge stabilizes the intermediate and makes it less destabilized compared to the intermediate formed at the ortho or para positions.
- Therefore, the intermediate carbonium ion formed after the initial attack of Br at the meta position is the least destabilized.
Statement c: Loss of aromaticity occurs when Br attacks at the ortho and para positions and not at the meta position.
- Aromatic compounds are characterized by having a delocalized system of π electrons, which provides stability to the molecule.
- When Br attacks at the ortho or para positions, the intermediate carbonium ion formed disrupts the delocalized π system.
- This loss of aromaticity is energetically unfavorable and makes the reaction less favorable.
- On the other hand, when Br attacks at the meta position, the delocalized π system remains intact, and hence, there is no loss of aromaticity.
- Therefore, loss of aromaticity occurs when Br attacks at the ortho and para positions and not at the meta position.
Statement d: It is easier to lose H to regain aromaticity from the meta position than from the ortho and para positions.
- After the initial attack of Br at the meta position, the intermediate carbonium ion can lose a proton (H+) to regenerate the aromaticity of the benzene ring.
- This loss of H+ is facilitated by the higher electron density at the meta position, which stabilizes the negative charge on the resulting intermediate.
- In contrast, at the ortho and para positions, the loss of H+ is less favorable due to the lower electron density and the destabilizing effect of the neighboring electron-withdrawing nitro group.
- Therefore, it is easier to lose H to regain aromaticity from the meta position than from the ortho and para positions.
To summarize, the correct statements are A and D. The higher electron density on the meta carbon, the least destabilized intermediate carbonium ion, the loss of aromaticity at the ortho and para positions, and the easier loss of H+ from the meta position all contribute to the preferential formation of m-bromonitrobenzene in the reaction of nit
Statement a: The electron density on the meta carbon is more than that on the ortho and para positions.
- In the presence of FeBr3, the bromination of nitrobenzene occurs via electrophilic aromatic substitution.
- FeBr3 acts as a Lewis acid and forms a complex with Br2, which enhances the electrophilicity of Br2.
- The nitro group (-NO2) is a strong electron-withdrawing group, which decreases the electron density on the aromatic ring.
- As a result, the meta position, which is further away from the nitro group, has a higher electron density compared to the ortho and para positions.
- Therefore, the electrophilic bromine (Br+) attacks the meta position more readily due to the higher electron density.
Statement b: The intermediate carbonium ion formed after the initial attack of Br at the meta position is least destabilized.
- When Br+ attacks the meta position, it forms a resonance-stabilized intermediate carbonium ion.
- The resonance structures of the intermediate carbonium ion show that the positive charge is delocalized over the meta carbon and the adjacent carbon atoms.
- This delocalization of positive charge stabilizes the intermediate and makes it less destabilized compared to the intermediate formed at the ortho or para positions.
- Therefore, the intermediate carbonium ion formed after the initial attack of Br at the meta position is the least destabilized.
Statement c: Loss of aromaticity occurs when Br attacks at the ortho and para positions and not at the meta position.
- Aromatic compounds are characterized by having a delocalized system of π electrons, which provides stability to the molecule.
- When Br attacks at the ortho or para positions, the intermediate carbonium ion formed disrupts the delocalized π system.
- This loss of aromaticity is energetically unfavorable and makes the reaction less favorable.
- On the other hand, when Br attacks at the meta position, the delocalized π system remains intact, and hence, there is no loss of aromaticity.
- Therefore, loss of aromaticity occurs when Br attacks at the ortho and para positions and not at the meta position.
Statement d: It is easier to lose H to regain aromaticity from the meta position than from the ortho and para positions.
- After the initial attack of Br at the meta position, the intermediate carbonium ion can lose a proton (H+) to regenerate the aromaticity of the benzene ring.
- This loss of H+ is facilitated by the higher electron density at the meta position, which stabilizes the negative charge on the resulting intermediate.
- In contrast, at the ortho and para positions, the loss of H+ is less favorable due to the lower electron density and the destabilizing effect of the neighboring electron-withdrawing nitro group.
- Therefore, it is easier to lose H to regain aromaticity from the meta position than from the ortho and para positions.
To summarize, the correct statements are A and D. The higher electron density on the meta carbon, the least destabilized intermediate carbonium ion, the loss of aromaticity at the ortho and para positions, and the easier loss of H+ from the meta position all contribute to the preferential formation of m-bromonitrobenzene in the reaction of nit

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
When nitrobenzene is treated with Br2 in presence of FeBr 3, the major product formed is m-bromonitrobenzene.Statements which are related to obtain the m-isomer area)The electron density on meta carbon is more than that on ortho and para positionsb)The intermediate carbonium ion formed after initial attack of Br+ at the meta position is least destabilisedc)Loss of aromaticity when Br+ attacks at the ortho and para positions and not at meta positiond)Easier loss of H+ to regain aromaticity from the meta position than from ortho and para positions.Correct answer is option 'A,D'. Can you explain this answer?
Question Description
When nitrobenzene is treated with Br2 in presence of FeBr 3, the major product formed is m-bromonitrobenzene.Statements which are related to obtain the m-isomer area)The electron density on meta carbon is more than that on ortho and para positionsb)The intermediate carbonium ion formed after initial attack of Br+ at the meta position is least destabilisedc)Loss of aromaticity when Br+ attacks at the ortho and para positions and not at meta positiond)Easier loss of H+ to regain aromaticity from the meta position than from ortho and para positions.Correct answer is option 'A,D'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about When nitrobenzene is treated with Br2 in presence of FeBr 3, the major product formed is m-bromonitrobenzene.Statements which are related to obtain the m-isomer area)The electron density on meta carbon is more than that on ortho and para positionsb)The intermediate carbonium ion formed after initial attack of Br+ at the meta position is least destabilisedc)Loss of aromaticity when Br+ attacks at the ortho and para positions and not at meta positiond)Easier loss of H+ to regain aromaticity from the meta position than from ortho and para positions.Correct answer is option 'A,D'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for When nitrobenzene is treated with Br2 in presence of FeBr 3, the major product formed is m-bromonitrobenzene.Statements which are related to obtain the m-isomer area)The electron density on meta carbon is more than that on ortho and para positionsb)The intermediate carbonium ion formed after initial attack of Br+ at the meta position is least destabilisedc)Loss of aromaticity when Br+ attacks at the ortho and para positions and not at meta positiond)Easier loss of H+ to regain aromaticity from the meta position than from ortho and para positions.Correct answer is option 'A,D'. Can you explain this answer?.
When nitrobenzene is treated with Br2 in presence of FeBr 3, the major product formed is m-bromonitrobenzene.Statements which are related to obtain the m-isomer area)The electron density on meta carbon is more than that on ortho and para positionsb)The intermediate carbonium ion formed after initial attack of Br+ at the meta position is least destabilisedc)Loss of aromaticity when Br+ attacks at the ortho and para positions and not at meta positiond)Easier loss of H+ to regain aromaticity from the meta position than from ortho and para positions.Correct answer is option 'A,D'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about When nitrobenzene is treated with Br2 in presence of FeBr 3, the major product formed is m-bromonitrobenzene.Statements which are related to obtain the m-isomer area)The electron density on meta carbon is more than that on ortho and para positionsb)The intermediate carbonium ion formed after initial attack of Br+ at the meta position is least destabilisedc)Loss of aromaticity when Br+ attacks at the ortho and para positions and not at meta positiond)Easier loss of H+ to regain aromaticity from the meta position than from ortho and para positions.Correct answer is option 'A,D'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for When nitrobenzene is treated with Br2 in presence of FeBr 3, the major product formed is m-bromonitrobenzene.Statements which are related to obtain the m-isomer area)The electron density on meta carbon is more than that on ortho and para positionsb)The intermediate carbonium ion formed after initial attack of Br+ at the meta position is least destabilisedc)Loss of aromaticity when Br+ attacks at the ortho and para positions and not at meta positiond)Easier loss of H+ to regain aromaticity from the meta position than from ortho and para positions.Correct answer is option 'A,D'. Can you explain this answer?.
Solutions for When nitrobenzene is treated with Br2 in presence of FeBr 3, the major product formed is m-bromonitrobenzene.Statements which are related to obtain the m-isomer area)The electron density on meta carbon is more than that on ortho and para positionsb)The intermediate carbonium ion formed after initial attack of Br+ at the meta position is least destabilisedc)Loss of aromaticity when Br+ attacks at the ortho and para positions and not at meta positiond)Easier loss of H+ to regain aromaticity from the meta position than from ortho and para positions.Correct answer is option 'A,D'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of When nitrobenzene is treated with Br2 in presence of FeBr 3, the major product formed is m-bromonitrobenzene.Statements which are related to obtain the m-isomer area)The electron density on meta carbon is more than that on ortho and para positionsb)The intermediate carbonium ion formed after initial attack of Br+ at the meta position is least destabilisedc)Loss of aromaticity when Br+ attacks at the ortho and para positions and not at meta positiond)Easier loss of H+ to regain aromaticity from the meta position than from ortho and para positions.Correct answer is option 'A,D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
When nitrobenzene is treated with Br2 in presence of FeBr 3, the major product formed is m-bromonitrobenzene.Statements which are related to obtain the m-isomer area)The electron density on meta carbon is more than that on ortho and para positionsb)The intermediate carbonium ion formed after initial attack of Br+ at the meta position is least destabilisedc)Loss of aromaticity when Br+ attacks at the ortho and para positions and not at meta positiond)Easier loss of H+ to regain aromaticity from the meta position than from ortho and para positions.Correct answer is option 'A,D'. Can you explain this answer?, a detailed solution for When nitrobenzene is treated with Br2 in presence of FeBr 3, the major product formed is m-bromonitrobenzene.Statements which are related to obtain the m-isomer area)The electron density on meta carbon is more than that on ortho and para positionsb)The intermediate carbonium ion formed after initial attack of Br+ at the meta position is least destabilisedc)Loss of aromaticity when Br+ attacks at the ortho and para positions and not at meta positiond)Easier loss of H+ to regain aromaticity from the meta position than from ortho and para positions.Correct answer is option 'A,D'. Can you explain this answer? has been provided alongside types of When nitrobenzene is treated with Br2 in presence of FeBr 3, the major product formed is m-bromonitrobenzene.Statements which are related to obtain the m-isomer area)The electron density on meta carbon is more than that on ortho and para positionsb)The intermediate carbonium ion formed after initial attack of Br+ at the meta position is least destabilisedc)Loss of aromaticity when Br+ attacks at the ortho and para positions and not at meta positiond)Easier loss of H+ to regain aromaticity from the meta position than from ortho and para positions.Correct answer is option 'A,D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice When nitrobenzene is treated with Br2 in presence of FeBr 3, the major product formed is m-bromonitrobenzene.Statements which are related to obtain the m-isomer area)The electron density on meta carbon is more than that on ortho and para positionsb)The intermediate carbonium ion formed after initial attack of Br+ at the meta position is least destabilisedc)Loss of aromaticity when Br+ attacks at the ortho and para positions and not at meta positiond)Easier loss of H+ to regain aromaticity from the meta position than from ortho and para positions.Correct answer is option 'A,D'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























