JEE Exam > JEE Questions > PARAGRAPH 1When 100 mL of 1.0 M HCl was mixed...
Start Learning for Free
PARAGRAPH 1
When 100 mL of 1.0 M HCl was mixed with 100 mL of 1.0 M NaOH in an insulated beaker at constant pressure, a temperature increase of 5.7 °C was measured for the beaker and its contents (Expt.1).
Because the enthalpy of neutralization of a strong acid with a strong base is a constant (–57.0 kJ mol–1), this experiment could be used to measure the calorimeter constant. In a second experiment (Expt. 2), 100 mL of 2.0 M acetic acid (Ka = 2.0 × 10–5) was mixed with 100 mL of 1.0 M NaOH (under identical conditions to Expt. 1) where a temperature rise of 5.6 °C was measured. (Consider heat capacity of all solutions as 4.2 J g–1 K–1 and density of all solutions as 1.0 g mL–1)
Because the enthalpy of neutralization of a strong acid with a strong base is a constant (–57.0 kJ mol–1), this experiment could be used to measure the calorimeter constant. In a second experiment (Expt. 2), 100 mL of 2.0 M acetic acid (Ka = 2.0 × 10–5) was mixed with 100 mL of 1.0 M NaOH (under identical conditions to Expt. 1) where a temperature rise of 5.6 °C was measured. (Consider heat capacity of all solutions as 4.2 J g–1 K–1 and density of all solutions as 1.0 g mL–1)
Q. Enthalpy of dissociation (in kJ mol–1) of acetic acid obtained from the Expt.2 is
- a)1.0
- b)10.0
- c)24.5
- d)51.4
Correct answer is option 'A'. Can you explain this answer?
Verified Answer
PARAGRAPH 1When 100 mL of 1.0 M HCl was mixed with 100 mL of 1.0 M NaO...
Let the heat capacity of insulated beaker be C.
Mass of aqueous content in expt. 1 = (100 + 100) × 1 = 200 g
⇒ ± Total heat capacity = (C + 200 × 4.2) J/K Moles of acid, base neutralised in expt. 1 = 0.1 × 1 = 0.1
⇒ Heat released in expt. 1 = 0.1 × 57 = 5.7 KJ = 5.7 × 1000 J
⇒ 5.7 × 1000 = (C + 200 × 4.2) × Δ´T.
5.7 × 1000 = (C + 200 + 4.2) × 5.7
⇒ (C + 200 × 4.2) = 1000
In second experiment,
Mass of aqueous content in expt. 1 = (100 + 100) × 1 = 200 g
⇒ ± Total heat capacity = (C + 200 × 4.2) J/K Moles of acid, base neutralised in expt. 1 = 0.1 × 1 = 0.1
⇒ Heat released in expt. 1 = 0.1 × 57 = 5.7 KJ = 5.7 × 1000 J
⇒ 5.7 × 1000 = (C + 200 × 4.2) × Δ´T.
5.7 × 1000 = (C + 200 + 4.2) × 5.7
⇒ (C + 200 × 4.2) = 1000
In second experiment,
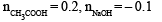
Total mass of aqueous content = 200 g
⇒ Total heat capacity = (C + 200 × 4.2) = 1000
⇒ Heat released = 1000 × 5.6 = 5600 J.
Overall, only 0.1 mol of CH3COOH undergo neutralization.
Overall, only 0.1 mol of CH3COOH undergo neutralization.
⇒ Δ Hneutralization of CH3COOH = -5600/0.1
= – 56000 J/mol
= – 56 KJ/mol.
= – 56 KJ/mol.
⇒ Δ Hneutralization of CH3COOH = 57 – 56 = 1 KJ/mol
Most Upvoted Answer
PARAGRAPH 1When 100 mL of 1.0 M HCl was mixed with 100 mL of 1.0 M NaO...
°C was observed. This is an example of an exothermic reaction, where heat is released as a result of the chemical reaction taking place. In this case, the acid and base react to form water and a salt. The temperature increase is a result of the energy released during the reaction. This type of reaction is commonly used in industries such as pharmaceuticals, food processing, and chemical manufacturing. Understanding the thermodynamics of these reactions is important for optimizing reaction conditions and improving efficiency.
Free Test
FREE
| Start Free Test |
Community Answer
PARAGRAPH 1When 100 mL of 1.0 M HCl was mixed with 100 mL of 1.0 M NaO...
1.0

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
PARAGRAPH 1When 100 mL of 1.0 M HCl was mixed with 100 mL of 1.0 M NaOH in an insulated beaker at constant pressure, a temperature increase of 5.7 °C was measured for the beaker and its contents (Expt.1).Because the enthalpy of neutralization of a strong acid with a strong base is a constant (–57.0 kJ mol–1), this experiment could be used to measure the calorimeter constant. In a second experiment (Expt. 2), 100 mL of 2.0 M acetic acid (Ka = 2.0 × 10–5) was mixed with 100 mL of 1.0 M NaOH (under identical conditions to Expt. 1) where a temperature rise of 5.6 °C was measured. (Consider heat capacity of all solutions as 4.2 J g–1 K–1 and density of all solutions as 1.0 g mL–1)Q.Enthalpy of dissociation (in kJ mol–1) of acetic acid obtained from the Expt.2 isa)1.0b)10.0c)24.5d)51.4Correct answer is option 'A'. Can you explain this answer?
Question Description
PARAGRAPH 1When 100 mL of 1.0 M HCl was mixed with 100 mL of 1.0 M NaOH in an insulated beaker at constant pressure, a temperature increase of 5.7 °C was measured for the beaker and its contents (Expt.1).Because the enthalpy of neutralization of a strong acid with a strong base is a constant (–57.0 kJ mol–1), this experiment could be used to measure the calorimeter constant. In a second experiment (Expt. 2), 100 mL of 2.0 M acetic acid (Ka = 2.0 × 10–5) was mixed with 100 mL of 1.0 M NaOH (under identical conditions to Expt. 1) where a temperature rise of 5.6 °C was measured. (Consider heat capacity of all solutions as 4.2 J g–1 K–1 and density of all solutions as 1.0 g mL–1)Q.Enthalpy of dissociation (in kJ mol–1) of acetic acid obtained from the Expt.2 isa)1.0b)10.0c)24.5d)51.4Correct answer is option 'A'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about PARAGRAPH 1When 100 mL of 1.0 M HCl was mixed with 100 mL of 1.0 M NaOH in an insulated beaker at constant pressure, a temperature increase of 5.7 °C was measured for the beaker and its contents (Expt.1).Because the enthalpy of neutralization of a strong acid with a strong base is a constant (–57.0 kJ mol–1), this experiment could be used to measure the calorimeter constant. In a second experiment (Expt. 2), 100 mL of 2.0 M acetic acid (Ka = 2.0 × 10–5) was mixed with 100 mL of 1.0 M NaOH (under identical conditions to Expt. 1) where a temperature rise of 5.6 °C was measured. (Consider heat capacity of all solutions as 4.2 J g–1 K–1 and density of all solutions as 1.0 g mL–1)Q.Enthalpy of dissociation (in kJ mol–1) of acetic acid obtained from the Expt.2 isa)1.0b)10.0c)24.5d)51.4Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for PARAGRAPH 1When 100 mL of 1.0 M HCl was mixed with 100 mL of 1.0 M NaOH in an insulated beaker at constant pressure, a temperature increase of 5.7 °C was measured for the beaker and its contents (Expt.1).Because the enthalpy of neutralization of a strong acid with a strong base is a constant (–57.0 kJ mol–1), this experiment could be used to measure the calorimeter constant. In a second experiment (Expt. 2), 100 mL of 2.0 M acetic acid (Ka = 2.0 × 10–5) was mixed with 100 mL of 1.0 M NaOH (under identical conditions to Expt. 1) where a temperature rise of 5.6 °C was measured. (Consider heat capacity of all solutions as 4.2 J g–1 K–1 and density of all solutions as 1.0 g mL–1)Q.Enthalpy of dissociation (in kJ mol–1) of acetic acid obtained from the Expt.2 isa)1.0b)10.0c)24.5d)51.4Correct answer is option 'A'. Can you explain this answer?.
PARAGRAPH 1When 100 mL of 1.0 M HCl was mixed with 100 mL of 1.0 M NaOH in an insulated beaker at constant pressure, a temperature increase of 5.7 °C was measured for the beaker and its contents (Expt.1).Because the enthalpy of neutralization of a strong acid with a strong base is a constant (–57.0 kJ mol–1), this experiment could be used to measure the calorimeter constant. In a second experiment (Expt. 2), 100 mL of 2.0 M acetic acid (Ka = 2.0 × 10–5) was mixed with 100 mL of 1.0 M NaOH (under identical conditions to Expt. 1) where a temperature rise of 5.6 °C was measured. (Consider heat capacity of all solutions as 4.2 J g–1 K–1 and density of all solutions as 1.0 g mL–1)Q.Enthalpy of dissociation (in kJ mol–1) of acetic acid obtained from the Expt.2 isa)1.0b)10.0c)24.5d)51.4Correct answer is option 'A'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about PARAGRAPH 1When 100 mL of 1.0 M HCl was mixed with 100 mL of 1.0 M NaOH in an insulated beaker at constant pressure, a temperature increase of 5.7 °C was measured for the beaker and its contents (Expt.1).Because the enthalpy of neutralization of a strong acid with a strong base is a constant (–57.0 kJ mol–1), this experiment could be used to measure the calorimeter constant. In a second experiment (Expt. 2), 100 mL of 2.0 M acetic acid (Ka = 2.0 × 10–5) was mixed with 100 mL of 1.0 M NaOH (under identical conditions to Expt. 1) where a temperature rise of 5.6 °C was measured. (Consider heat capacity of all solutions as 4.2 J g–1 K–1 and density of all solutions as 1.0 g mL–1)Q.Enthalpy of dissociation (in kJ mol–1) of acetic acid obtained from the Expt.2 isa)1.0b)10.0c)24.5d)51.4Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for PARAGRAPH 1When 100 mL of 1.0 M HCl was mixed with 100 mL of 1.0 M NaOH in an insulated beaker at constant pressure, a temperature increase of 5.7 °C was measured for the beaker and its contents (Expt.1).Because the enthalpy of neutralization of a strong acid with a strong base is a constant (–57.0 kJ mol–1), this experiment could be used to measure the calorimeter constant. In a second experiment (Expt. 2), 100 mL of 2.0 M acetic acid (Ka = 2.0 × 10–5) was mixed with 100 mL of 1.0 M NaOH (under identical conditions to Expt. 1) where a temperature rise of 5.6 °C was measured. (Consider heat capacity of all solutions as 4.2 J g–1 K–1 and density of all solutions as 1.0 g mL–1)Q.Enthalpy of dissociation (in kJ mol–1) of acetic acid obtained from the Expt.2 isa)1.0b)10.0c)24.5d)51.4Correct answer is option 'A'. Can you explain this answer?.
Solutions for PARAGRAPH 1When 100 mL of 1.0 M HCl was mixed with 100 mL of 1.0 M NaOH in an insulated beaker at constant pressure, a temperature increase of 5.7 °C was measured for the beaker and its contents (Expt.1).Because the enthalpy of neutralization of a strong acid with a strong base is a constant (–57.0 kJ mol–1), this experiment could be used to measure the calorimeter constant. In a second experiment (Expt. 2), 100 mL of 2.0 M acetic acid (Ka = 2.0 × 10–5) was mixed with 100 mL of 1.0 M NaOH (under identical conditions to Expt. 1) where a temperature rise of 5.6 °C was measured. (Consider heat capacity of all solutions as 4.2 J g–1 K–1 and density of all solutions as 1.0 g mL–1)Q.Enthalpy of dissociation (in kJ mol–1) of acetic acid obtained from the Expt.2 isa)1.0b)10.0c)24.5d)51.4Correct answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of PARAGRAPH 1When 100 mL of 1.0 M HCl was mixed with 100 mL of 1.0 M NaOH in an insulated beaker at constant pressure, a temperature increase of 5.7 °C was measured for the beaker and its contents (Expt.1).Because the enthalpy of neutralization of a strong acid with a strong base is a constant (–57.0 kJ mol–1), this experiment could be used to measure the calorimeter constant. In a second experiment (Expt. 2), 100 mL of 2.0 M acetic acid (Ka = 2.0 × 10–5) was mixed with 100 mL of 1.0 M NaOH (under identical conditions to Expt. 1) where a temperature rise of 5.6 °C was measured. (Consider heat capacity of all solutions as 4.2 J g–1 K–1 and density of all solutions as 1.0 g mL–1)Q.Enthalpy of dissociation (in kJ mol–1) of acetic acid obtained from the Expt.2 isa)1.0b)10.0c)24.5d)51.4Correct answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
PARAGRAPH 1When 100 mL of 1.0 M HCl was mixed with 100 mL of 1.0 M NaOH in an insulated beaker at constant pressure, a temperature increase of 5.7 °C was measured for the beaker and its contents (Expt.1).Because the enthalpy of neutralization of a strong acid with a strong base is a constant (–57.0 kJ mol–1), this experiment could be used to measure the calorimeter constant. In a second experiment (Expt. 2), 100 mL of 2.0 M acetic acid (Ka = 2.0 × 10–5) was mixed with 100 mL of 1.0 M NaOH (under identical conditions to Expt. 1) where a temperature rise of 5.6 °C was measured. (Consider heat capacity of all solutions as 4.2 J g–1 K–1 and density of all solutions as 1.0 g mL–1)Q.Enthalpy of dissociation (in kJ mol–1) of acetic acid obtained from the Expt.2 isa)1.0b)10.0c)24.5d)51.4Correct answer is option 'A'. Can you explain this answer?, a detailed solution for PARAGRAPH 1When 100 mL of 1.0 M HCl was mixed with 100 mL of 1.0 M NaOH in an insulated beaker at constant pressure, a temperature increase of 5.7 °C was measured for the beaker and its contents (Expt.1).Because the enthalpy of neutralization of a strong acid with a strong base is a constant (–57.0 kJ mol–1), this experiment could be used to measure the calorimeter constant. In a second experiment (Expt. 2), 100 mL of 2.0 M acetic acid (Ka = 2.0 × 10–5) was mixed with 100 mL of 1.0 M NaOH (under identical conditions to Expt. 1) where a temperature rise of 5.6 °C was measured. (Consider heat capacity of all solutions as 4.2 J g–1 K–1 and density of all solutions as 1.0 g mL–1)Q.Enthalpy of dissociation (in kJ mol–1) of acetic acid obtained from the Expt.2 isa)1.0b)10.0c)24.5d)51.4Correct answer is option 'A'. Can you explain this answer? has been provided alongside types of PARAGRAPH 1When 100 mL of 1.0 M HCl was mixed with 100 mL of 1.0 M NaOH in an insulated beaker at constant pressure, a temperature increase of 5.7 °C was measured for the beaker and its contents (Expt.1).Because the enthalpy of neutralization of a strong acid with a strong base is a constant (–57.0 kJ mol–1), this experiment could be used to measure the calorimeter constant. In a second experiment (Expt. 2), 100 mL of 2.0 M acetic acid (Ka = 2.0 × 10–5) was mixed with 100 mL of 1.0 M NaOH (under identical conditions to Expt. 1) where a temperature rise of 5.6 °C was measured. (Consider heat capacity of all solutions as 4.2 J g–1 K–1 and density of all solutions as 1.0 g mL–1)Q.Enthalpy of dissociation (in kJ mol–1) of acetic acid obtained from the Expt.2 isa)1.0b)10.0c)24.5d)51.4Correct answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice PARAGRAPH 1When 100 mL of 1.0 M HCl was mixed with 100 mL of 1.0 M NaOH in an insulated beaker at constant pressure, a temperature increase of 5.7 °C was measured for the beaker and its contents (Expt.1).Because the enthalpy of neutralization of a strong acid with a strong base is a constant (–57.0 kJ mol–1), this experiment could be used to measure the calorimeter constant. In a second experiment (Expt. 2), 100 mL of 2.0 M acetic acid (Ka = 2.0 × 10–5) was mixed with 100 mL of 1.0 M NaOH (under identical conditions to Expt. 1) where a temperature rise of 5.6 °C was measured. (Consider heat capacity of all solutions as 4.2 J g–1 K–1 and density of all solutions as 1.0 g mL–1)Q.Enthalpy of dissociation (in kJ mol–1) of acetic acid obtained from the Expt.2 isa)1.0b)10.0c)24.5d)51.4Correct answer is option 'A'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


























