JEE Exam > JEE Questions > At 25°C, the solubility product of Mg(OH)...
Start Learning for Free
At 25°C, the solubility product of Mg(OH)2 is 1.0 × 10–11. At which pH, will Mg2+ ions start precipitating in the form of Mg(OH)2 from a solution of 0.001 M Mg2+ ions?
- a)9
- b)10
- c)11
- d)8
Correct answer is option 'B'. Can you explain this answer?
Verified Answer
At 25°C, the solubility product of Mg(OH)2 is 1.0 × 10&ndash...
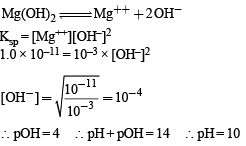
Most Upvoted Answer
At 25°C, the solubility product of Mg(OH)2 is 1.0 × 10&ndash...
At 25, many people are in the early stages of their careers and are often focused on building their professional skills and establishing themselves in their chosen fields. They may still be exploring different career paths and may be open to taking on new challenges and opportunities.
Financially, individuals at 25 may be starting to earn a steady income and are typically more independent than they were in their early 20s. They may be paying off student loans, saving for the future, and possibly considering big financial decisions such as buying a home or investing in their retirement.
In terms of personal relationships, some individuals at 25 may be in committed relationships or getting married, while others may still be exploring and dating. Their social circles may consist of a mix of friends from college, work, and other activities.
At this age, people often have a good amount of energy and may still enjoy going out and socializing. They may also be interested in hobbies and activities that align with their personal interests and passions.
Overall, being 25 is a time of transition and growth, where individuals are working towards establishing themselves professionally, managing their finances, and navigating personal relationships.
Financially, individuals at 25 may be starting to earn a steady income and are typically more independent than they were in their early 20s. They may be paying off student loans, saving for the future, and possibly considering big financial decisions such as buying a home or investing in their retirement.
In terms of personal relationships, some individuals at 25 may be in committed relationships or getting married, while others may still be exploring and dating. Their social circles may consist of a mix of friends from college, work, and other activities.
At this age, people often have a good amount of energy and may still enjoy going out and socializing. They may also be interested in hobbies and activities that align with their personal interests and passions.
Overall, being 25 is a time of transition and growth, where individuals are working towards establishing themselves professionally, managing their finances, and navigating personal relationships.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
At 25°C, the solubility product of Mg(OH)2 is 1.0 × 10–11. At which pH, will Mg2+ ions start precipitating in the form of Mg(OH)2 from a solution of 0.001 M Mg2+ ions?a)9b)10c)11d)8Correct answer is option 'B'. Can you explain this answer?
Question Description
At 25°C, the solubility product of Mg(OH)2 is 1.0 × 10–11. At which pH, will Mg2+ ions start precipitating in the form of Mg(OH)2 from a solution of 0.001 M Mg2+ ions?a)9b)10c)11d)8Correct answer is option 'B'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about At 25°C, the solubility product of Mg(OH)2 is 1.0 × 10–11. At which pH, will Mg2+ ions start precipitating in the form of Mg(OH)2 from a solution of 0.001 M Mg2+ ions?a)9b)10c)11d)8Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for At 25°C, the solubility product of Mg(OH)2 is 1.0 × 10–11. At which pH, will Mg2+ ions start precipitating in the form of Mg(OH)2 from a solution of 0.001 M Mg2+ ions?a)9b)10c)11d)8Correct answer is option 'B'. Can you explain this answer?.
At 25°C, the solubility product of Mg(OH)2 is 1.0 × 10–11. At which pH, will Mg2+ ions start precipitating in the form of Mg(OH)2 from a solution of 0.001 M Mg2+ ions?a)9b)10c)11d)8Correct answer is option 'B'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about At 25°C, the solubility product of Mg(OH)2 is 1.0 × 10–11. At which pH, will Mg2+ ions start precipitating in the form of Mg(OH)2 from a solution of 0.001 M Mg2+ ions?a)9b)10c)11d)8Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for At 25°C, the solubility product of Mg(OH)2 is 1.0 × 10–11. At which pH, will Mg2+ ions start precipitating in the form of Mg(OH)2 from a solution of 0.001 M Mg2+ ions?a)9b)10c)11d)8Correct answer is option 'B'. Can you explain this answer?.
Solutions for At 25°C, the solubility product of Mg(OH)2 is 1.0 × 10–11. At which pH, will Mg2+ ions start precipitating in the form of Mg(OH)2 from a solution of 0.001 M Mg2+ ions?a)9b)10c)11d)8Correct answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of At 25°C, the solubility product of Mg(OH)2 is 1.0 × 10–11. At which pH, will Mg2+ ions start precipitating in the form of Mg(OH)2 from a solution of 0.001 M Mg2+ ions?a)9b)10c)11d)8Correct answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
At 25°C, the solubility product of Mg(OH)2 is 1.0 × 10–11. At which pH, will Mg2+ ions start precipitating in the form of Mg(OH)2 from a solution of 0.001 M Mg2+ ions?a)9b)10c)11d)8Correct answer is option 'B'. Can you explain this answer?, a detailed solution for At 25°C, the solubility product of Mg(OH)2 is 1.0 × 10–11. At which pH, will Mg2+ ions start precipitating in the form of Mg(OH)2 from a solution of 0.001 M Mg2+ ions?a)9b)10c)11d)8Correct answer is option 'B'. Can you explain this answer? has been provided alongside types of At 25°C, the solubility product of Mg(OH)2 is 1.0 × 10–11. At which pH, will Mg2+ ions start precipitating in the form of Mg(OH)2 from a solution of 0.001 M Mg2+ ions?a)9b)10c)11d)8Correct answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice At 25°C, the solubility product of Mg(OH)2 is 1.0 × 10–11. At which pH, will Mg2+ ions start precipitating in the form of Mg(OH)2 from a solution of 0.001 M Mg2+ ions?a)9b)10c)11d)8Correct answer is option 'B'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























