JEE Exam > JEE Questions > The synthesis of alkyl fluorides is best acco...
Start Learning for Free
The synthesis of alkyl fluorides is best accomplished by : [JEE M 2015]
- a)Finkelstein reaction
- b)Swarts reaction
- c)Free radical fluorination
- d)Sandmeyer's reaction
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
The synthesis of alkyl fluorides is best accomplished by: [JEE M 20...
Correct Option (B) Swarts reaction
Explanation:
Alkyl fluorides can be prepared by action of mercurous fluoride or antimony trifluorides (inorganic fluorides) on corresponding alkyl halide. This reaction is known as Swarts reaction.
Explanation:
Alkyl fluorides can be prepared by action of mercurous fluoride or antimony trifluorides (inorganic fluorides) on corresponding alkyl halide. This reaction is known as Swarts reaction.
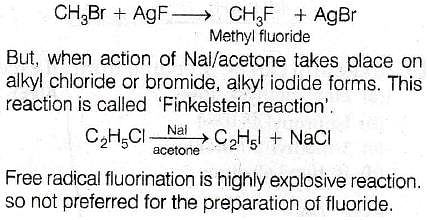
Most Upvoted Answer
The synthesis of alkyl fluorides is best accomplished by: [JEE M 20...
Swarts Reaction for Synthesis of Alkyl Fluorides
The Swarts reaction is a method for the synthesis of alkyl fluorides. It involves the conversion of alkyl chlorides or bromides to alkyl fluorides by the action of a fluoride source such as HF or metal fluorides. Here's how the Swarts reaction works:
Key Points:
- Alkyl chlorides or bromides are treated with a fluoride source such as HF or metal fluorides.
- The reaction proceeds via a nucleophilic substitution mechanism where the chlorine or bromine atom is replaced by a fluorine atom.
- The use of HF as a fluoride source can lead to side reactions and is not the preferred method.
- Metal fluorides such as AgF or SbF3 are commonly used as they provide better control and higher yields.
Advantages of Swarts Reaction:
- It is a straightforward method for the synthesis of alkyl fluorides.
- Metal fluorides provide better selectivity and yield compared to HF.
- The reaction can be easily scaled up for industrial applications.
Limitations of Swarts Reaction:
- The Swarts reaction can be limited by the availability of suitable starting materials.
- Care must be taken to avoid side reactions and to ensure the desired product is obtained.
In conclusion, the Swarts reaction is a reliable method for the synthesis of alkyl fluorides from alkyl chlorides or bromides. It offers good control over the reaction and can be scaled up for industrial use.
The Swarts reaction is a method for the synthesis of alkyl fluorides. It involves the conversion of alkyl chlorides or bromides to alkyl fluorides by the action of a fluoride source such as HF or metal fluorides. Here's how the Swarts reaction works:
Key Points:
- Alkyl chlorides or bromides are treated with a fluoride source such as HF or metal fluorides.
- The reaction proceeds via a nucleophilic substitution mechanism where the chlorine or bromine atom is replaced by a fluorine atom.
- The use of HF as a fluoride source can lead to side reactions and is not the preferred method.
- Metal fluorides such as AgF or SbF3 are commonly used as they provide better control and higher yields.
Advantages of Swarts Reaction:
- It is a straightforward method for the synthesis of alkyl fluorides.
- Metal fluorides provide better selectivity and yield compared to HF.
- The reaction can be easily scaled up for industrial applications.
Limitations of Swarts Reaction:
- The Swarts reaction can be limited by the availability of suitable starting materials.
- Care must be taken to avoid side reactions and to ensure the desired product is obtained.
In conclusion, the Swarts reaction is a reliable method for the synthesis of alkyl fluorides from alkyl chlorides or bromides. It offers good control over the reaction and can be scaled up for industrial use.
Attention JEE Students!
To make sure you are not studying endlessly, EduRev has designed JEE study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in JEE.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
The synthesis of alkyl fluorides is best accomplished by: [JEE M 2015]a)Finkelstein reactionb)Swarts reactionc)Free radical fluorinationd)Sandmeyer's reactionCorrect answer is option 'B'. Can you explain this answer?
Question Description
The synthesis of alkyl fluorides is best accomplished by: [JEE M 2015]a)Finkelstein reactionb)Swarts reactionc)Free radical fluorinationd)Sandmeyer's reactionCorrect answer is option 'B'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about The synthesis of alkyl fluorides is best accomplished by: [JEE M 2015]a)Finkelstein reactionb)Swarts reactionc)Free radical fluorinationd)Sandmeyer's reactionCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The synthesis of alkyl fluorides is best accomplished by: [JEE M 2015]a)Finkelstein reactionb)Swarts reactionc)Free radical fluorinationd)Sandmeyer's reactionCorrect answer is option 'B'. Can you explain this answer?.
The synthesis of alkyl fluorides is best accomplished by: [JEE M 2015]a)Finkelstein reactionb)Swarts reactionc)Free radical fluorinationd)Sandmeyer's reactionCorrect answer is option 'B'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about The synthesis of alkyl fluorides is best accomplished by: [JEE M 2015]a)Finkelstein reactionb)Swarts reactionc)Free radical fluorinationd)Sandmeyer's reactionCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The synthesis of alkyl fluorides is best accomplished by: [JEE M 2015]a)Finkelstein reactionb)Swarts reactionc)Free radical fluorinationd)Sandmeyer's reactionCorrect answer is option 'B'. Can you explain this answer?.
Solutions for The synthesis of alkyl fluorides is best accomplished by: [JEE M 2015]a)Finkelstein reactionb)Swarts reactionc)Free radical fluorinationd)Sandmeyer's reactionCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of The synthesis of alkyl fluorides is best accomplished by: [JEE M 2015]a)Finkelstein reactionb)Swarts reactionc)Free radical fluorinationd)Sandmeyer's reactionCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The synthesis of alkyl fluorides is best accomplished by: [JEE M 2015]a)Finkelstein reactionb)Swarts reactionc)Free radical fluorinationd)Sandmeyer's reactionCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for The synthesis of alkyl fluorides is best accomplished by: [JEE M 2015]a)Finkelstein reactionb)Swarts reactionc)Free radical fluorinationd)Sandmeyer's reactionCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of The synthesis of alkyl fluorides is best accomplished by: [JEE M 2015]a)Finkelstein reactionb)Swarts reactionc)Free radical fluorinationd)Sandmeyer's reactionCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The synthesis of alkyl fluorides is best accomplished by: [JEE M 2015]a)Finkelstein reactionb)Swarts reactionc)Free radical fluorinationd)Sandmeyer's reactionCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























