JEE Exam > JEE Questions > The oxidation of SO2 by O2 to SO3 is an exoth...
Start Learning for Free
The oxidation of SO2 by O2 to SO3 is an exothermic reaction. The yield of SO3 will be maximum if
- a)temperature is increased and pressure is kept constant
- b)temperature is reduced and pressure is increased
- c)both temperature and pressure are increased
- d)both temperature and pressure are reduced
Correct answer is option 'B'. Can you explain this answer?
Verified Answer
The oxidation of SO2 by O2 to SO3 is an exothermic reaction. The yield...
TIPS/Formulae :
(i) According is Le-Chateliers principle, exothermic, reactions are favoured at low temperature.
(ii) According to Le-Chateliers principle, the reaction in which n < 0, are favoured at high pressure.
(i) According is Le-Chateliers principle, exothermic, reactions are favoured at low temperature.
(ii) According to Le-Chateliers principle, the reaction in which n < 0, are favoured at high pressure.
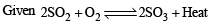
∵ It is exothermic reaction
∴Yield of SO3 is maximum at low temperature
n = 2 – 3= – 1 or n < 0
∴ Yield of SO3 is maximum at high pressure.
∴Yield of SO3 is maximum at low temperature
n = 2 – 3= – 1 or n < 0
∴ Yield of SO3 is maximum at high pressure.
Most Upvoted Answer
The oxidation of SO2 by O2 to SO3 is an exothermic reaction. The yield...
The oxidation of SO2 by O2 to SO3 is an exothermic reaction. The yield of SO3 will be maximum if:
Explanation:
The oxidation of SO2 by O2 to form SO3 is an exothermic reaction, which means it releases heat. The yield of SO3 refers to the amount of SO3 produced in the reaction. To maximize the yield of SO3, we need to consider the effect of temperature and pressure on the reaction.
Effect of Temperature:
When temperature is increased, the rate of reaction generally increases. However, for an exothermic reaction, increasing the temperature will shift the equilibrium towards the reactants, in this case, SO2 and O2. This is because the increase in temperature favors the endothermic direction of the reaction, which is the reverse reaction. As a result, the yield of SO3 will decrease.
Effect of Pressure:
Increasing the pressure generally favors the formation of products. In this case, increasing the pressure will shift the equilibrium towards the products, which is the formation of SO3. This is because the formation of SO3 involves a decrease in the number of moles of gas compared to the reactants, SO2 and O2. Increasing the pressure helps to counteract the decrease in volume, favoring the formation of SO3. Therefore, increasing the pressure will increase the yield of SO3.
Conclusion:
Based on the above explanations, the yield of SO3 will be maximum if the temperature is reduced and the pressure is increased. Reducing the temperature helps to shift the equilibrium towards the products, while increasing the pressure helps to favor the formation of SO3 by counteracting the decrease in volume.
Explanation:
The oxidation of SO2 by O2 to form SO3 is an exothermic reaction, which means it releases heat. The yield of SO3 refers to the amount of SO3 produced in the reaction. To maximize the yield of SO3, we need to consider the effect of temperature and pressure on the reaction.
Effect of Temperature:
When temperature is increased, the rate of reaction generally increases. However, for an exothermic reaction, increasing the temperature will shift the equilibrium towards the reactants, in this case, SO2 and O2. This is because the increase in temperature favors the endothermic direction of the reaction, which is the reverse reaction. As a result, the yield of SO3 will decrease.
Effect of Pressure:
Increasing the pressure generally favors the formation of products. In this case, increasing the pressure will shift the equilibrium towards the products, which is the formation of SO3. This is because the formation of SO3 involves a decrease in the number of moles of gas compared to the reactants, SO2 and O2. Increasing the pressure helps to counteract the decrease in volume, favoring the formation of SO3. Therefore, increasing the pressure will increase the yield of SO3.
Conclusion:
Based on the above explanations, the yield of SO3 will be maximum if the temperature is reduced and the pressure is increased. Reducing the temperature helps to shift the equilibrium towards the products, while increasing the pressure helps to favor the formation of SO3 by counteracting the decrease in volume.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
The oxidation of SO2 by O2 to SO3 is an exothermic reaction. The yield of SO3 will be maximum ifa)temperature is increased and pressure is kept constantb)temperature is reduced and pressure is increasedc)both temperature and pressure are increasedd)both temperature and pressure are reducedCorrect answer is option 'B'. Can you explain this answer?
Question Description
The oxidation of SO2 by O2 to SO3 is an exothermic reaction. The yield of SO3 will be maximum ifa)temperature is increased and pressure is kept constantb)temperature is reduced and pressure is increasedc)both temperature and pressure are increasedd)both temperature and pressure are reducedCorrect answer is option 'B'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about The oxidation of SO2 by O2 to SO3 is an exothermic reaction. The yield of SO3 will be maximum ifa)temperature is increased and pressure is kept constantb)temperature is reduced and pressure is increasedc)both temperature and pressure are increasedd)both temperature and pressure are reducedCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The oxidation of SO2 by O2 to SO3 is an exothermic reaction. The yield of SO3 will be maximum ifa)temperature is increased and pressure is kept constantb)temperature is reduced and pressure is increasedc)both temperature and pressure are increasedd)both temperature and pressure are reducedCorrect answer is option 'B'. Can you explain this answer?.
The oxidation of SO2 by O2 to SO3 is an exothermic reaction. The yield of SO3 will be maximum ifa)temperature is increased and pressure is kept constantb)temperature is reduced and pressure is increasedc)both temperature and pressure are increasedd)both temperature and pressure are reducedCorrect answer is option 'B'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about The oxidation of SO2 by O2 to SO3 is an exothermic reaction. The yield of SO3 will be maximum ifa)temperature is increased and pressure is kept constantb)temperature is reduced and pressure is increasedc)both temperature and pressure are increasedd)both temperature and pressure are reducedCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The oxidation of SO2 by O2 to SO3 is an exothermic reaction. The yield of SO3 will be maximum ifa)temperature is increased and pressure is kept constantb)temperature is reduced and pressure is increasedc)both temperature and pressure are increasedd)both temperature and pressure are reducedCorrect answer is option 'B'. Can you explain this answer?.
Solutions for The oxidation of SO2 by O2 to SO3 is an exothermic reaction. The yield of SO3 will be maximum ifa)temperature is increased and pressure is kept constantb)temperature is reduced and pressure is increasedc)both temperature and pressure are increasedd)both temperature and pressure are reducedCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of The oxidation of SO2 by O2 to SO3 is an exothermic reaction. The yield of SO3 will be maximum ifa)temperature is increased and pressure is kept constantb)temperature is reduced and pressure is increasedc)both temperature and pressure are increasedd)both temperature and pressure are reducedCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The oxidation of SO2 by O2 to SO3 is an exothermic reaction. The yield of SO3 will be maximum ifa)temperature is increased and pressure is kept constantb)temperature is reduced and pressure is increasedc)both temperature and pressure are increasedd)both temperature and pressure are reducedCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for The oxidation of SO2 by O2 to SO3 is an exothermic reaction. The yield of SO3 will be maximum ifa)temperature is increased and pressure is kept constantb)temperature is reduced and pressure is increasedc)both temperature and pressure are increasedd)both temperature and pressure are reducedCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of The oxidation of SO2 by O2 to SO3 is an exothermic reaction. The yield of SO3 will be maximum ifa)temperature is increased and pressure is kept constantb)temperature is reduced and pressure is increasedc)both temperature and pressure are increasedd)both temperature and pressure are reducedCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The oxidation of SO2 by O2 to SO3 is an exothermic reaction. The yield of SO3 will be maximum ifa)temperature is increased and pressure is kept constantb)temperature is reduced and pressure is increasedc)both temperature and pressure are increasedd)both temperature and pressure are reducedCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























