JEE Exam > JEE Questions > A gas described by van der Waals equation &nd...
Start Learning for Free
A gas described by van der Waals equation – (2008- 1 Mark)
- a)behave similar to an ideal gas in the limit of large molar volumes
- b)behaves similar to an ideal gas is in limit of large pressures
- c)is characterised by van der Waals coefficients that are dependent on the identity of the gas but are independent of the temperature.
- d)has the pressure that is lower than the pressure exerted by the same gas behaving ideally
Correct answer is option 'A,C'. Can you explain this answer?
Verified Answer
A gas described by van der Waals equation – (2008- 1 Mark)a)be...
Vander Waals equation is
 = nRT [For n moles of a gas)
= nRT [For n moles of a gas)a, b are vander Waals constants The ideal gas equation is PV = nRT [For n moles of a gas] where P is pressure excerted by ideal gas and V is volume occupied by ideal gas.
In vander Waals equation the term represents the pressure exerted by the gas and (V– nb) the volume occupied by the gas. At low pressure, when the gas occoupies large volume the intermolecular distance between gaseous moleculas is quite large and in such case there is no significant role played by intermolecular forces and thus the gas behaves like an ideal gas thus (a) is correct NOTE : Under high pressure the intermolecular distance decreases and the intermolecular forces play a significant role and the gas shows a devation from ideal behaviour.
represents the pressure exerted by the gas and (V– nb) the volume occupied by the gas. At low pressure, when the gas occoupies large volume the intermolecular distance between gaseous moleculas is quite large and in such case there is no significant role played by intermolecular forces and thus the gas behaves like an ideal gas thus (a) is correct NOTE : Under high pressure the intermolecular distance decreases and the intermolecular forces play a significant role and the gas shows a devation from ideal behaviour.
Thus (b) is not correct. a, b i.e. the vander Waals coefficients defined on the nature of gas and are independent of temperature so (c) is correct.
The pressure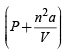 is not lower than P so (d) is
is not lower than P so (d) is
not correct.
Hence the correct anser is (a, c).
In vander Waals equation the term
 represents the pressure exerted by the gas and (V– nb) the volume occupied by the gas. At low pressure, when the gas occoupies large volume the intermolecular distance between gaseous moleculas is quite large and in such case there is no significant role played by intermolecular forces and thus the gas behaves like an ideal gas thus (a) is correct NOTE : Under high pressure the intermolecular distance decreases and the intermolecular forces play a significant role and the gas shows a devation from ideal behaviour.
represents the pressure exerted by the gas and (V– nb) the volume occupied by the gas. At low pressure, when the gas occoupies large volume the intermolecular distance between gaseous moleculas is quite large and in such case there is no significant role played by intermolecular forces and thus the gas behaves like an ideal gas thus (a) is correct NOTE : Under high pressure the intermolecular distance decreases and the intermolecular forces play a significant role and the gas shows a devation from ideal behaviour.Thus (b) is not correct. a, b i.e. the vander Waals coefficients defined on the nature of gas and are independent of temperature so (c) is correct.
The pressure
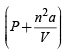 is not lower than P so (d) is
is not lower than P so (d) isnot correct.
Hence the correct anser is (a, c).
Most Upvoted Answer
A gas described by van der Waals equation – (2008- 1 Mark)a)be...
Behavior of gas described by van der Waals equation:
Gas described by van der Waals equation exhibit the following behaviors:
- Similar to an ideal gas in the limit of large molar volumes: At high molar volumes, the volume occupied by gas molecules becomes negligible compared to the total volume, and the intermolecular forces can be ignored. In this limit, the gas behaves similar to an ideal gas.
- Characterized by van der Waals coefficients dependent on the identity of the gas: The van der Waals coefficients (a and b) in the van der Waals equation are specific to each gas and depend on the nature of intermolecular forces for that particular gas. These coefficients are independent of temperature but vary with the identity of the gas.
Therefore, option A and C are correct as gas described by van der Waals equation behaves similar to an ideal gas in the limit of large molar volumes and the van der Waals coefficients are dependent on the identity of the gas.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
A gas described by van der Waals equation – (2008- 1 Mark)a)behave similar to an ideal gas in the limit of large molar volumesb)behaves similar to an ideal gas is in limit of large pressuresc)is characterised by van der Waals coefficients that are dependent on the identity of the gas but are independent of the temperature.d)has the pressure that is lower than the pressure exerted by the same gas behaving ideallyCorrect answer is option 'A,C'. Can you explain this answer?
Question Description
A gas described by van der Waals equation – (2008- 1 Mark)a)behave similar to an ideal gas in the limit of large molar volumesb)behaves similar to an ideal gas is in limit of large pressuresc)is characterised by van der Waals coefficients that are dependent on the identity of the gas but are independent of the temperature.d)has the pressure that is lower than the pressure exerted by the same gas behaving ideallyCorrect answer is option 'A,C'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about A gas described by van der Waals equation – (2008- 1 Mark)a)behave similar to an ideal gas in the limit of large molar volumesb)behaves similar to an ideal gas is in limit of large pressuresc)is characterised by van der Waals coefficients that are dependent on the identity of the gas but are independent of the temperature.d)has the pressure that is lower than the pressure exerted by the same gas behaving ideallyCorrect answer is option 'A,C'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for A gas described by van der Waals equation – (2008- 1 Mark)a)behave similar to an ideal gas in the limit of large molar volumesb)behaves similar to an ideal gas is in limit of large pressuresc)is characterised by van der Waals coefficients that are dependent on the identity of the gas but are independent of the temperature.d)has the pressure that is lower than the pressure exerted by the same gas behaving ideallyCorrect answer is option 'A,C'. Can you explain this answer?.
A gas described by van der Waals equation – (2008- 1 Mark)a)behave similar to an ideal gas in the limit of large molar volumesb)behaves similar to an ideal gas is in limit of large pressuresc)is characterised by van der Waals coefficients that are dependent on the identity of the gas but are independent of the temperature.d)has the pressure that is lower than the pressure exerted by the same gas behaving ideallyCorrect answer is option 'A,C'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about A gas described by van der Waals equation – (2008- 1 Mark)a)behave similar to an ideal gas in the limit of large molar volumesb)behaves similar to an ideal gas is in limit of large pressuresc)is characterised by van der Waals coefficients that are dependent on the identity of the gas but are independent of the temperature.d)has the pressure that is lower than the pressure exerted by the same gas behaving ideallyCorrect answer is option 'A,C'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for A gas described by van der Waals equation – (2008- 1 Mark)a)behave similar to an ideal gas in the limit of large molar volumesb)behaves similar to an ideal gas is in limit of large pressuresc)is characterised by van der Waals coefficients that are dependent on the identity of the gas but are independent of the temperature.d)has the pressure that is lower than the pressure exerted by the same gas behaving ideallyCorrect answer is option 'A,C'. Can you explain this answer?.
Solutions for A gas described by van der Waals equation – (2008- 1 Mark)a)behave similar to an ideal gas in the limit of large molar volumesb)behaves similar to an ideal gas is in limit of large pressuresc)is characterised by van der Waals coefficients that are dependent on the identity of the gas but are independent of the temperature.d)has the pressure that is lower than the pressure exerted by the same gas behaving ideallyCorrect answer is option 'A,C'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of A gas described by van der Waals equation – (2008- 1 Mark)a)behave similar to an ideal gas in the limit of large molar volumesb)behaves similar to an ideal gas is in limit of large pressuresc)is characterised by van der Waals coefficients that are dependent on the identity of the gas but are independent of the temperature.d)has the pressure that is lower than the pressure exerted by the same gas behaving ideallyCorrect answer is option 'A,C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
A gas described by van der Waals equation – (2008- 1 Mark)a)behave similar to an ideal gas in the limit of large molar volumesb)behaves similar to an ideal gas is in limit of large pressuresc)is characterised by van der Waals coefficients that are dependent on the identity of the gas but are independent of the temperature.d)has the pressure that is lower than the pressure exerted by the same gas behaving ideallyCorrect answer is option 'A,C'. Can you explain this answer?, a detailed solution for A gas described by van der Waals equation – (2008- 1 Mark)a)behave similar to an ideal gas in the limit of large molar volumesb)behaves similar to an ideal gas is in limit of large pressuresc)is characterised by van der Waals coefficients that are dependent on the identity of the gas but are independent of the temperature.d)has the pressure that is lower than the pressure exerted by the same gas behaving ideallyCorrect answer is option 'A,C'. Can you explain this answer? has been provided alongside types of A gas described by van der Waals equation – (2008- 1 Mark)a)behave similar to an ideal gas in the limit of large molar volumesb)behaves similar to an ideal gas is in limit of large pressuresc)is characterised by van der Waals coefficients that are dependent on the identity of the gas but are independent of the temperature.d)has the pressure that is lower than the pressure exerted by the same gas behaving ideallyCorrect answer is option 'A,C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice A gas described by van der Waals equation – (2008- 1 Mark)a)behave similar to an ideal gas in the limit of large molar volumesb)behaves similar to an ideal gas is in limit of large pressuresc)is characterised by van der Waals coefficients that are dependent on the identity of the gas but are independent of the temperature.d)has the pressure that is lower than the pressure exerted by the same gas behaving ideallyCorrect answer is option 'A,C'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























