IIT JAM Exam > IIT JAM Questions > In the following statements, the correct stat...
Start Learning for Free
In the following statements, the correct statement (s) is/are
- a)2P3/2 is the ground state term symbol of F
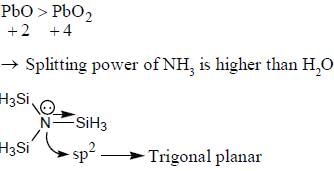
- b)PbO is more stable than PbO2
- c)The power of NH3 to split the metal d-orbitals is higher than H2O
- d)The central atom of N(SiH3)3 is sp3 hybridised
Correct answer is option 'A,B,C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
In the following statements, the correct statement (s) is/area)2P3/2 i...
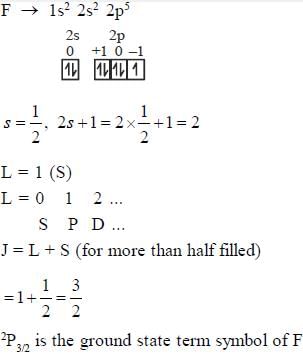
→ Stability of lower oxidation state becomes more stable on moving down the ground because of inert pair effect
Most Upvoted Answer
In the following statements, the correct statement (s) is/area)2P3/2 i...
Statement (a): 2P3/2 is the ground state term symbol of F
The term symbol is used to describe the electronic state of an atom or molecule. It consists of a combination of letters and numbers that represent the total angular momentum, total spin, and orbital angular momentum of the system. The term symbol is derived from the electron configuration of the atom or molecule.
In the case of fluorine (F), the electron configuration is 1s2 2s2 2p5. The ground state of fluorine has one unpaired electron in the 2p orbital. The total angular momentum (L) for this electron is 1, and the total spin (S) is 1/2. Therefore, the term symbol for the ground state of fluorine is 2P3/2.
Statement (b): PbO is more stable than PbO2
Lead (Pb) can exhibit a +2 or +4 oxidation state. PbO and PbO2 are two common oxides of lead.
PbO has Pb in the +2 oxidation state, while PbO2 has Pb in the +4 oxidation state. Generally, lower oxidation states are more stable than higher oxidation states for most elements. This is because lower oxidation states require less energy to achieve.
In the case of lead, the +2 oxidation state is more stable than the +4 oxidation state. Therefore, PbO is more stable than PbO2.
Statement (c): The power of NH3 to split the metal d-orbitals is higher than H2O
Ammonia (NH3) and water (H2O) are both commonly used as ligands in coordination compounds. Ligands can donate electron pairs to a metal center, forming coordination bonds.
Ammonia has a lone pair of electrons on the nitrogen atom, which can be donated to a metal center. This lone pair can interact with the metal d-orbitals, causing them to split into different energy levels. This splitting is known as ligand field splitting.
Water also has a lone pair of electrons on the oxygen atom, but the electronegativity of oxygen is higher than that of nitrogen. This means that the oxygen atom in water is less willing to donate its lone pair to the metal center compared to the nitrogen atom in ammonia.
Therefore, the power of NH3 to split the metal d-orbitals is higher than H2O.
Statement (d): The central atom of N(SiH3)3 is sp3 hybridized
The central atom in N(SiH3)3 is nitrogen (N). Nitrogen has five valence electrons and can form three sigma bonds with the three SiH3 groups in the molecule.
In order to accommodate these three sigma bonds, as well as the lone pair of electrons on nitrogen, the nitrogen atom undergoes sp3 hybridization. This results in four sp3 hybrid orbitals, three of which are used to form sigma bonds with the SiH3 groups and one of which contains the lone pair of electrons.
Therefore, the central atom of N(SiH3)3 is sp3 hybridized.
The term symbol is used to describe the electronic state of an atom or molecule. It consists of a combination of letters and numbers that represent the total angular momentum, total spin, and orbital angular momentum of the system. The term symbol is derived from the electron configuration of the atom or molecule.
In the case of fluorine (F), the electron configuration is 1s2 2s2 2p5. The ground state of fluorine has one unpaired electron in the 2p orbital. The total angular momentum (L) for this electron is 1, and the total spin (S) is 1/2. Therefore, the term symbol for the ground state of fluorine is 2P3/2.
Statement (b): PbO is more stable than PbO2
Lead (Pb) can exhibit a +2 or +4 oxidation state. PbO and PbO2 are two common oxides of lead.
PbO has Pb in the +2 oxidation state, while PbO2 has Pb in the +4 oxidation state. Generally, lower oxidation states are more stable than higher oxidation states for most elements. This is because lower oxidation states require less energy to achieve.
In the case of lead, the +2 oxidation state is more stable than the +4 oxidation state. Therefore, PbO is more stable than PbO2.
Statement (c): The power of NH3 to split the metal d-orbitals is higher than H2O
Ammonia (NH3) and water (H2O) are both commonly used as ligands in coordination compounds. Ligands can donate electron pairs to a metal center, forming coordination bonds.
Ammonia has a lone pair of electrons on the nitrogen atom, which can be donated to a metal center. This lone pair can interact with the metal d-orbitals, causing them to split into different energy levels. This splitting is known as ligand field splitting.
Water also has a lone pair of electrons on the oxygen atom, but the electronegativity of oxygen is higher than that of nitrogen. This means that the oxygen atom in water is less willing to donate its lone pair to the metal center compared to the nitrogen atom in ammonia.
Therefore, the power of NH3 to split the metal d-orbitals is higher than H2O.
Statement (d): The central atom of N(SiH3)3 is sp3 hybridized
The central atom in N(SiH3)3 is nitrogen (N). Nitrogen has five valence electrons and can form three sigma bonds with the three SiH3 groups in the molecule.
In order to accommodate these three sigma bonds, as well as the lone pair of electrons on nitrogen, the nitrogen atom undergoes sp3 hybridization. This results in four sp3 hybrid orbitals, three of which are used to form sigma bonds with the SiH3 groups and one of which contains the lone pair of electrons.
Therefore, the central atom of N(SiH3)3 is sp3 hybridized.

|
Explore Courses for IIT JAM exam
|

|
Similar IIT JAM Doubts
In the following statements, the correct statement (s) is/area)2P3/2 is the ground state term symbol of Fb)PbO is more stable than PbO2c)The power of NH3 to split the metal d-orbitals is higher than H2Od)The central atom of N(SiH3)3 is sp3 hybridisedCorrect answer is option 'A,B,C'. Can you explain this answer?
Question Description
In the following statements, the correct statement (s) is/area)2P3/2 is the ground state term symbol of Fb)PbO is more stable than PbO2c)The power of NH3 to split the metal d-orbitals is higher than H2Od)The central atom of N(SiH3)3 is sp3 hybridisedCorrect answer is option 'A,B,C'. Can you explain this answer? for IIT JAM 2024 is part of IIT JAM preparation. The Question and answers have been prepared according to the IIT JAM exam syllabus. Information about In the following statements, the correct statement (s) is/area)2P3/2 is the ground state term symbol of Fb)PbO is more stable than PbO2c)The power of NH3 to split the metal d-orbitals is higher than H2Od)The central atom of N(SiH3)3 is sp3 hybridisedCorrect answer is option 'A,B,C'. Can you explain this answer? covers all topics & solutions for IIT JAM 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In the following statements, the correct statement (s) is/area)2P3/2 is the ground state term symbol of Fb)PbO is more stable than PbO2c)The power of NH3 to split the metal d-orbitals is higher than H2Od)The central atom of N(SiH3)3 is sp3 hybridisedCorrect answer is option 'A,B,C'. Can you explain this answer?.
In the following statements, the correct statement (s) is/area)2P3/2 is the ground state term symbol of Fb)PbO is more stable than PbO2c)The power of NH3 to split the metal d-orbitals is higher than H2Od)The central atom of N(SiH3)3 is sp3 hybridisedCorrect answer is option 'A,B,C'. Can you explain this answer? for IIT JAM 2024 is part of IIT JAM preparation. The Question and answers have been prepared according to the IIT JAM exam syllabus. Information about In the following statements, the correct statement (s) is/area)2P3/2 is the ground state term symbol of Fb)PbO is more stable than PbO2c)The power of NH3 to split the metal d-orbitals is higher than H2Od)The central atom of N(SiH3)3 is sp3 hybridisedCorrect answer is option 'A,B,C'. Can you explain this answer? covers all topics & solutions for IIT JAM 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In the following statements, the correct statement (s) is/area)2P3/2 is the ground state term symbol of Fb)PbO is more stable than PbO2c)The power of NH3 to split the metal d-orbitals is higher than H2Od)The central atom of N(SiH3)3 is sp3 hybridisedCorrect answer is option 'A,B,C'. Can you explain this answer?.
Solutions for In the following statements, the correct statement (s) is/area)2P3/2 is the ground state term symbol of Fb)PbO is more stable than PbO2c)The power of NH3 to split the metal d-orbitals is higher than H2Od)The central atom of N(SiH3)3 is sp3 hybridisedCorrect answer is option 'A,B,C'. Can you explain this answer? in English & in Hindi are available as part of our courses for IIT JAM.
Download more important topics, notes, lectures and mock test series for IIT JAM Exam by signing up for free.
Here you can find the meaning of In the following statements, the correct statement (s) is/area)2P3/2 is the ground state term symbol of Fb)PbO is more stable than PbO2c)The power of NH3 to split the metal d-orbitals is higher than H2Od)The central atom of N(SiH3)3 is sp3 hybridisedCorrect answer is option 'A,B,C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
In the following statements, the correct statement (s) is/area)2P3/2 is the ground state term symbol of Fb)PbO is more stable than PbO2c)The power of NH3 to split the metal d-orbitals is higher than H2Od)The central atom of N(SiH3)3 is sp3 hybridisedCorrect answer is option 'A,B,C'. Can you explain this answer?, a detailed solution for In the following statements, the correct statement (s) is/area)2P3/2 is the ground state term symbol of Fb)PbO is more stable than PbO2c)The power of NH3 to split the metal d-orbitals is higher than H2Od)The central atom of N(SiH3)3 is sp3 hybridisedCorrect answer is option 'A,B,C'. Can you explain this answer? has been provided alongside types of In the following statements, the correct statement (s) is/area)2P3/2 is the ground state term symbol of Fb)PbO is more stable than PbO2c)The power of NH3 to split the metal d-orbitals is higher than H2Od)The central atom of N(SiH3)3 is sp3 hybridisedCorrect answer is option 'A,B,C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice In the following statements, the correct statement (s) is/area)2P3/2 is the ground state term symbol of Fb)PbO is more stable than PbO2c)The power of NH3 to split the metal d-orbitals is higher than H2Od)The central atom of N(SiH3)3 is sp3 hybridisedCorrect answer is option 'A,B,C'. Can you explain this answer? tests, examples and also practice IIT JAM tests.

|
Explore Courses for IIT JAM exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















