IIT JAM Exam > IIT JAM Questions > Hemoglobin and myoglobin are two proteins wit...
Start Learning for Free
Hemoglobin and myoglobin are two proteins with high degree of similarity in the structure and function. Following are few statements regarding hemoglobin and myoglobin. Select the correct statement.
- a)Oxygen binding curve of myoglobin is hyperbolic in nature and that of hemoglobin is sigmoidal in nature.
- b)Myoglobin does not show positive cooperativity whereas hemoglobin shows negative cooperativity.
- c)Myoglobin and hemoglobin, both show positive cooperativity.
- d)Hemoglobin has high affinity for oxygen than myoglobin
Correct answer is option 'A'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Hemoglobin and myoglobin are two proteins with high degree of similari...
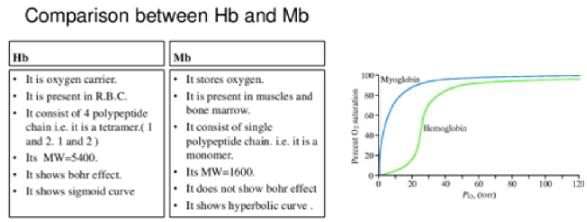
Hemoglobin’s oxygen binding affinity is relatively weak compared to myoglobin’s affinity for oxygen. Hemoglobin’s oxygen-binding curve is in the shape of a sigmoidal curve. In red blood cells, the oxygen-binding curve for hemoglobin displays an “S” shaped called a sigmoidal curve. A sigmoidal curve shows that oxygen binding is cooperative; that is, when one site binds oxygen, the probability that the remaining unoccupied sites that will bind to oxygen will increase.
The sigmoid shape is a consequence of the four subunits of hemoglobin “cooperating” in the binding of oxygen.
The myoglobin saturation curve is a rectangular hyperbola, NOT sigmoid. This different shape is the result of having a single subunit.
The single subunit character of myoglobin and the multiple subunit character of hemoglobin give each of these molecules their characteristic “behavior” with respect to oxygen binding and different physiological uses for which each is suited.
The sigmoid shape is a consequence of the four subunits of hemoglobin “cooperating” in the binding of oxygen.
The myoglobin saturation curve is a rectangular hyperbola, NOT sigmoid. This different shape is the result of having a single subunit.
The single subunit character of myoglobin and the multiple subunit character of hemoglobin give each of these molecules their characteristic “behavior” with respect to oxygen binding and different physiological uses for which each is suited.

Most Upvoted Answer
Hemoglobin and myoglobin are two proteins with high degree of similari...
Statement: Oxygen binding curve of myoglobin is hyperbolic in nature and that of hemoglobin is sigmoidal in nature.
Explanation:
Hemoglobin:
- Hemoglobin is a protein found in red blood cells and is responsible for carrying oxygen from the lungs to the tissues and organs of the body.
- Hemoglobin is a tetrameric protein, meaning it is made up of four subunits.
- Each subunit of hemoglobin contains a heme group, which is capable of binding to one oxygen molecule.
- The binding of oxygen to hemoglobin is cooperative, meaning that the binding of one oxygen molecule increases the affinity of the remaining subunits for oxygen.
- As a result of this cooperative binding, the oxygen binding curve of hemoglobin is sigmoidal in shape.
- At low oxygen concentrations, hemoglobin has a low affinity for oxygen, but as the concentration of oxygen increases, the affinity of hemoglobin for oxygen increases, resulting in a rapid increase in oxygen binding.
Myoglobin:
- Myoglobin is a protein found in muscle cells and is responsible for storing oxygen within the muscles.
- Myoglobin is a monomeric protein, meaning it is made up of a single subunit.
- Like hemoglobin, myoglobin contains a heme group that can bind to oxygen.
- However, unlike hemoglobin, myoglobin does not exhibit cooperative binding.
- The oxygen binding curve of myoglobin is hyperbolic in shape, meaning that as the concentration of oxygen increases, the rate of oxygen binding gradually decreases until it reaches a plateau.
- This hyperbolic shape indicates that myoglobin has a high affinity for oxygen, as it can bind to oxygen even at low concentrations.
Conclusion:
The correct statement is option 'A': Oxygen binding curve of myoglobin is hyperbolic in nature and that of hemoglobin is sigmoidal in nature.
Explanation:
Hemoglobin:
- Hemoglobin is a protein found in red blood cells and is responsible for carrying oxygen from the lungs to the tissues and organs of the body.
- Hemoglobin is a tetrameric protein, meaning it is made up of four subunits.
- Each subunit of hemoglobin contains a heme group, which is capable of binding to one oxygen molecule.
- The binding of oxygen to hemoglobin is cooperative, meaning that the binding of one oxygen molecule increases the affinity of the remaining subunits for oxygen.
- As a result of this cooperative binding, the oxygen binding curve of hemoglobin is sigmoidal in shape.
- At low oxygen concentrations, hemoglobin has a low affinity for oxygen, but as the concentration of oxygen increases, the affinity of hemoglobin for oxygen increases, resulting in a rapid increase in oxygen binding.
Myoglobin:
- Myoglobin is a protein found in muscle cells and is responsible for storing oxygen within the muscles.
- Myoglobin is a monomeric protein, meaning it is made up of a single subunit.
- Like hemoglobin, myoglobin contains a heme group that can bind to oxygen.
- However, unlike hemoglobin, myoglobin does not exhibit cooperative binding.
- The oxygen binding curve of myoglobin is hyperbolic in shape, meaning that as the concentration of oxygen increases, the rate of oxygen binding gradually decreases until it reaches a plateau.
- This hyperbolic shape indicates that myoglobin has a high affinity for oxygen, as it can bind to oxygen even at low concentrations.
Conclusion:
The correct statement is option 'A': Oxygen binding curve of myoglobin is hyperbolic in nature and that of hemoglobin is sigmoidal in nature.

|
Explore Courses for IIT JAM exam
|

|
Similar IIT JAM Doubts
Hemoglobin and myoglobin are two proteins with high degree of similarity in the structure and function. Following are few statements regarding hemoglobin and myoglobin. Select the correct statement.a)Oxygen binding curve of myoglobin is hyperbolic in nature and that of hemoglobin is sigmoidal in nature.b)Myoglobin does not show positive cooperativity whereas hemoglobin shows negative cooperativity.c)Myoglobin and hemoglobin, both show positive cooperativity.d)Hemoglobin has high affinity for oxygen than myoglobinCorrect answer is option 'A'. Can you explain this answer?
Question Description
Hemoglobin and myoglobin are two proteins with high degree of similarity in the structure and function. Following are few statements regarding hemoglobin and myoglobin. Select the correct statement.a)Oxygen binding curve of myoglobin is hyperbolic in nature and that of hemoglobin is sigmoidal in nature.b)Myoglobin does not show positive cooperativity whereas hemoglobin shows negative cooperativity.c)Myoglobin and hemoglobin, both show positive cooperativity.d)Hemoglobin has high affinity for oxygen than myoglobinCorrect answer is option 'A'. Can you explain this answer? for IIT JAM 2024 is part of IIT JAM preparation. The Question and answers have been prepared according to the IIT JAM exam syllabus. Information about Hemoglobin and myoglobin are two proteins with high degree of similarity in the structure and function. Following are few statements regarding hemoglobin and myoglobin. Select the correct statement.a)Oxygen binding curve of myoglobin is hyperbolic in nature and that of hemoglobin is sigmoidal in nature.b)Myoglobin does not show positive cooperativity whereas hemoglobin shows negative cooperativity.c)Myoglobin and hemoglobin, both show positive cooperativity.d)Hemoglobin has high affinity for oxygen than myoglobinCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for IIT JAM 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Hemoglobin and myoglobin are two proteins with high degree of similarity in the structure and function. Following are few statements regarding hemoglobin and myoglobin. Select the correct statement.a)Oxygen binding curve of myoglobin is hyperbolic in nature and that of hemoglobin is sigmoidal in nature.b)Myoglobin does not show positive cooperativity whereas hemoglobin shows negative cooperativity.c)Myoglobin and hemoglobin, both show positive cooperativity.d)Hemoglobin has high affinity for oxygen than myoglobinCorrect answer is option 'A'. Can you explain this answer?.
Hemoglobin and myoglobin are two proteins with high degree of similarity in the structure and function. Following are few statements regarding hemoglobin and myoglobin. Select the correct statement.a)Oxygen binding curve of myoglobin is hyperbolic in nature and that of hemoglobin is sigmoidal in nature.b)Myoglobin does not show positive cooperativity whereas hemoglobin shows negative cooperativity.c)Myoglobin and hemoglobin, both show positive cooperativity.d)Hemoglobin has high affinity for oxygen than myoglobinCorrect answer is option 'A'. Can you explain this answer? for IIT JAM 2024 is part of IIT JAM preparation. The Question and answers have been prepared according to the IIT JAM exam syllabus. Information about Hemoglobin and myoglobin are two proteins with high degree of similarity in the structure and function. Following are few statements regarding hemoglobin and myoglobin. Select the correct statement.a)Oxygen binding curve of myoglobin is hyperbolic in nature and that of hemoglobin is sigmoidal in nature.b)Myoglobin does not show positive cooperativity whereas hemoglobin shows negative cooperativity.c)Myoglobin and hemoglobin, both show positive cooperativity.d)Hemoglobin has high affinity for oxygen than myoglobinCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for IIT JAM 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Hemoglobin and myoglobin are two proteins with high degree of similarity in the structure and function. Following are few statements regarding hemoglobin and myoglobin. Select the correct statement.a)Oxygen binding curve of myoglobin is hyperbolic in nature and that of hemoglobin is sigmoidal in nature.b)Myoglobin does not show positive cooperativity whereas hemoglobin shows negative cooperativity.c)Myoglobin and hemoglobin, both show positive cooperativity.d)Hemoglobin has high affinity for oxygen than myoglobinCorrect answer is option 'A'. Can you explain this answer?.
Solutions for Hemoglobin and myoglobin are two proteins with high degree of similarity in the structure and function. Following are few statements regarding hemoglobin and myoglobin. Select the correct statement.a)Oxygen binding curve of myoglobin is hyperbolic in nature and that of hemoglobin is sigmoidal in nature.b)Myoglobin does not show positive cooperativity whereas hemoglobin shows negative cooperativity.c)Myoglobin and hemoglobin, both show positive cooperativity.d)Hemoglobin has high affinity for oxygen than myoglobinCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for IIT JAM.
Download more important topics, notes, lectures and mock test series for IIT JAM Exam by signing up for free.
Here you can find the meaning of Hemoglobin and myoglobin are two proteins with high degree of similarity in the structure and function. Following are few statements regarding hemoglobin and myoglobin. Select the correct statement.a)Oxygen binding curve of myoglobin is hyperbolic in nature and that of hemoglobin is sigmoidal in nature.b)Myoglobin does not show positive cooperativity whereas hemoglobin shows negative cooperativity.c)Myoglobin and hemoglobin, both show positive cooperativity.d)Hemoglobin has high affinity for oxygen than myoglobinCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Hemoglobin and myoglobin are two proteins with high degree of similarity in the structure and function. Following are few statements regarding hemoglobin and myoglobin. Select the correct statement.a)Oxygen binding curve of myoglobin is hyperbolic in nature and that of hemoglobin is sigmoidal in nature.b)Myoglobin does not show positive cooperativity whereas hemoglobin shows negative cooperativity.c)Myoglobin and hemoglobin, both show positive cooperativity.d)Hemoglobin has high affinity for oxygen than myoglobinCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for Hemoglobin and myoglobin are two proteins with high degree of similarity in the structure and function. Following are few statements regarding hemoglobin and myoglobin. Select the correct statement.a)Oxygen binding curve of myoglobin is hyperbolic in nature and that of hemoglobin is sigmoidal in nature.b)Myoglobin does not show positive cooperativity whereas hemoglobin shows negative cooperativity.c)Myoglobin and hemoglobin, both show positive cooperativity.d)Hemoglobin has high affinity for oxygen than myoglobinCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of Hemoglobin and myoglobin are two proteins with high degree of similarity in the structure and function. Following are few statements regarding hemoglobin and myoglobin. Select the correct statement.a)Oxygen binding curve of myoglobin is hyperbolic in nature and that of hemoglobin is sigmoidal in nature.b)Myoglobin does not show positive cooperativity whereas hemoglobin shows negative cooperativity.c)Myoglobin and hemoglobin, both show positive cooperativity.d)Hemoglobin has high affinity for oxygen than myoglobinCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Hemoglobin and myoglobin are two proteins with high degree of similarity in the structure and function. Following are few statements regarding hemoglobin and myoglobin. Select the correct statement.a)Oxygen binding curve of myoglobin is hyperbolic in nature and that of hemoglobin is sigmoidal in nature.b)Myoglobin does not show positive cooperativity whereas hemoglobin shows negative cooperativity.c)Myoglobin and hemoglobin, both show positive cooperativity.d)Hemoglobin has high affinity for oxygen than myoglobinCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice IIT JAM tests.

|
Explore Courses for IIT JAM exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















