Class 12 Exam > Class 12 Questions > When a hydrogen when a hydrogen atom emits ph...
Start Learning for Free
When a hydrogen when a hydrogen atom emits photon of energy 12.09eV its orbital angular momentum changes by?
Most Upvoted Answer
When a hydrogen when a hydrogen atom emits photon of energy 12.09eV it...
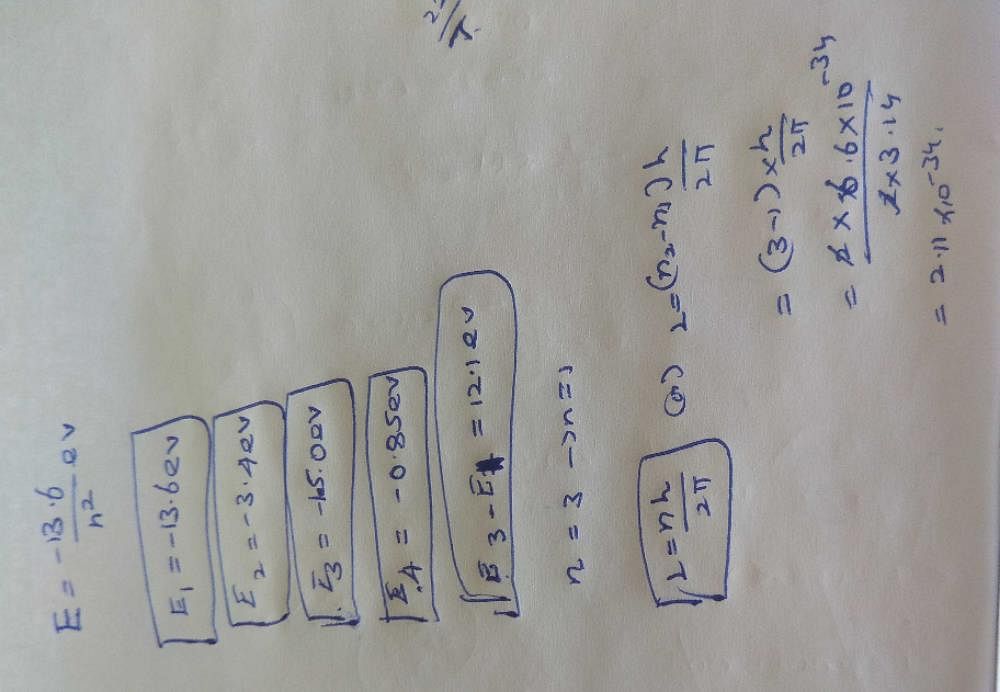
Community Answer
When a hydrogen when a hydrogen atom emits photon of energy 12.09eV it...
The Change in Orbital Angular Momentum of a Hydrogen Atom
When a hydrogen atom emits a photon of energy 12.09 eV, its orbital angular momentum changes. To understand this phenomenon, we need to explore the principles of quantum mechanics and the behavior of electrons in atoms.
1. Quantum Mechanics
Quantum mechanics is a branch of physics that describes the behavior of particles at the atomic and subatomic levels. It provides a framework for understanding the properties and interactions of matter and energy.
2. Energy Levels in Hydrogen Atom
In a hydrogen atom, the electron revolves around the nucleus in specific energy levels or orbitals. These energy levels are characterized by their principal quantum number, n, which determines the size and energy of the orbit.
3. Emission of Photons
When an electron transitions from a higher energy level to a lower one, it releases energy in the form of a photon. The energy of the emitted photon is equal to the difference in energy between the initial and final states of the electron.
4. Orbital Angular Momentum
The orbital angular momentum of an electron is a property that describes the rotational motion of the electron around the nucleus. It is quantized and depends on the principal quantum number, n, and the azimuthal quantum number, l.
5. Change in Orbital Angular Momentum
When a hydrogen atom emits a photon, the electron transitions from a higher energy level to a lower one. This transition leads to a change in the orbital angular momentum of the electron.
6. Calculation of the Change in Orbital Angular Momentum
To calculate the change in orbital angular momentum, we need to consider the initial and final states of the electron. The orbital angular momentum is given by the formula:
L = ħ√(l(l+1))
where ħ is the reduced Planck's constant and l is the azimuthal quantum number.
The change in orbital angular momentum can be obtained by subtracting the initial orbital angular momentum from the final orbital angular momentum.
ΔL = L_final - L_initial
7. Conclusion
In conclusion, when a hydrogen atom emits a photon of energy 12.09 eV, its orbital angular momentum changes. This change is a result of the transition of the electron from a higher energy level to a lower one. The calculation of the change in orbital angular momentum involves considering the initial and final states of the electron and applying the formula for orbital angular momentum.
When a hydrogen atom emits a photon of energy 12.09 eV, its orbital angular momentum changes. To understand this phenomenon, we need to explore the principles of quantum mechanics and the behavior of electrons in atoms.
1. Quantum Mechanics
Quantum mechanics is a branch of physics that describes the behavior of particles at the atomic and subatomic levels. It provides a framework for understanding the properties and interactions of matter and energy.
2. Energy Levels in Hydrogen Atom
In a hydrogen atom, the electron revolves around the nucleus in specific energy levels or orbitals. These energy levels are characterized by their principal quantum number, n, which determines the size and energy of the orbit.
3. Emission of Photons
When an electron transitions from a higher energy level to a lower one, it releases energy in the form of a photon. The energy of the emitted photon is equal to the difference in energy between the initial and final states of the electron.
4. Orbital Angular Momentum
The orbital angular momentum of an electron is a property that describes the rotational motion of the electron around the nucleus. It is quantized and depends on the principal quantum number, n, and the azimuthal quantum number, l.
5. Change in Orbital Angular Momentum
When a hydrogen atom emits a photon, the electron transitions from a higher energy level to a lower one. This transition leads to a change in the orbital angular momentum of the electron.
6. Calculation of the Change in Orbital Angular Momentum
To calculate the change in orbital angular momentum, we need to consider the initial and final states of the electron. The orbital angular momentum is given by the formula:
L = ħ√(l(l+1))
where ħ is the reduced Planck's constant and l is the azimuthal quantum number.
The change in orbital angular momentum can be obtained by subtracting the initial orbital angular momentum from the final orbital angular momentum.
ΔL = L_final - L_initial
7. Conclusion
In conclusion, when a hydrogen atom emits a photon of energy 12.09 eV, its orbital angular momentum changes. This change is a result of the transition of the electron from a higher energy level to a lower one. The calculation of the change in orbital angular momentum involves considering the initial and final states of the electron and applying the formula for orbital angular momentum.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
When a hydrogen when a hydrogen atom emits photon of energy 12.09eV its orbital angular momentum changes by?
Question Description
When a hydrogen when a hydrogen atom emits photon of energy 12.09eV its orbital angular momentum changes by? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about When a hydrogen when a hydrogen atom emits photon of energy 12.09eV its orbital angular momentum changes by? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for When a hydrogen when a hydrogen atom emits photon of energy 12.09eV its orbital angular momentum changes by?.
When a hydrogen when a hydrogen atom emits photon of energy 12.09eV its orbital angular momentum changes by? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about When a hydrogen when a hydrogen atom emits photon of energy 12.09eV its orbital angular momentum changes by? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for When a hydrogen when a hydrogen atom emits photon of energy 12.09eV its orbital angular momentum changes by?.
Solutions for When a hydrogen when a hydrogen atom emits photon of energy 12.09eV its orbital angular momentum changes by? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of When a hydrogen when a hydrogen atom emits photon of energy 12.09eV its orbital angular momentum changes by? defined & explained in the simplest way possible. Besides giving the explanation of
When a hydrogen when a hydrogen atom emits photon of energy 12.09eV its orbital angular momentum changes by?, a detailed solution for When a hydrogen when a hydrogen atom emits photon of energy 12.09eV its orbital angular momentum changes by? has been provided alongside types of When a hydrogen when a hydrogen atom emits photon of energy 12.09eV its orbital angular momentum changes by? theory, EduRev gives you an
ample number of questions to practice When a hydrogen when a hydrogen atom emits photon of energy 12.09eV its orbital angular momentum changes by? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.



















