Chemistry Exam > Chemistry Questions > If the ionization and electron gain enthalpy ...
Start Learning for Free
If the ionization and electron gain enthalpy of an element are 275 and 86 Kcalmol−1 respectively, then the electronegativity of the element on the Mulliken scale is:
- a)3.16
- b)0.0
- c)2.8
- d)4.16
Correct answer is option 'C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
If the ionization and electron gain enthalpy of an element are 275and8...
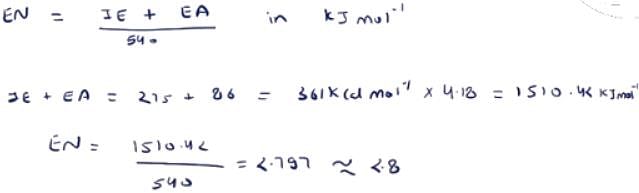
Most Upvoted Answer
If the ionization and electron gain enthalpy of an element are 275and8...
-1 respectively, then the element is:
The given ionization enthalpy and electron gain enthalpy are not sufficient to identify the element uniquely. We need to compare these values with the values of ionization enthalpy and electron gain enthalpy of known elements.
However, we can make the following observations:
1. The ionization enthalpy of the element is relatively high (275 Kcal/mol), indicating that it requires a considerable amount of energy to remove an electron from the outermost shell of the atom.
2. The electron gain enthalpy of the element is relatively low (86 Kcal/mol), indicating that the atom does not readily accept an additional electron to form a negative ion.
Based on these observations, we can tentatively conclude that the element is likely to be a metal or a metalloid, rather than a non-metal. Metals generally have low electron gain enthalpies, while non-metals have high electron gain enthalpies.
However, without more information, we cannot determine the identity of the element with certainty. We would need additional data, such as the atomic number or the electronic configuration, to make a definitive identification.
The given ionization enthalpy and electron gain enthalpy are not sufficient to identify the element uniquely. We need to compare these values with the values of ionization enthalpy and electron gain enthalpy of known elements.
However, we can make the following observations:
1. The ionization enthalpy of the element is relatively high (275 Kcal/mol), indicating that it requires a considerable amount of energy to remove an electron from the outermost shell of the atom.
2. The electron gain enthalpy of the element is relatively low (86 Kcal/mol), indicating that the atom does not readily accept an additional electron to form a negative ion.
Based on these observations, we can tentatively conclude that the element is likely to be a metal or a metalloid, rather than a non-metal. Metals generally have low electron gain enthalpies, while non-metals have high electron gain enthalpies.
However, without more information, we cannot determine the identity of the element with certainty. We would need additional data, such as the atomic number or the electronic configuration, to make a definitive identification.
Free Test
FREE
| Start Free Test |
Community Answer
If the ionization and electron gain enthalpy of an element are 275and8...
How did Ei become 1150.6 and Ea become 359.8

|
Explore Courses for Chemistry exam
|

|
Similar Chemistry Doubts
If the ionization and electron gain enthalpy of an element are 275and86Kcalmol−1 respectively, then the electronegativity of the element on the Mulliken scale is:a)3.16b)0.0c)2.8d)4.16Correct answer is option 'C'. Can you explain this answer?
Question Description
If the ionization and electron gain enthalpy of an element are 275and86Kcalmol−1 respectively, then the electronegativity of the element on the Mulliken scale is:a)3.16b)0.0c)2.8d)4.16Correct answer is option 'C'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about If the ionization and electron gain enthalpy of an element are 275and86Kcalmol−1 respectively, then the electronegativity of the element on the Mulliken scale is:a)3.16b)0.0c)2.8d)4.16Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for If the ionization and electron gain enthalpy of an element are 275and86Kcalmol−1 respectively, then the electronegativity of the element on the Mulliken scale is:a)3.16b)0.0c)2.8d)4.16Correct answer is option 'C'. Can you explain this answer?.
If the ionization and electron gain enthalpy of an element are 275and86Kcalmol−1 respectively, then the electronegativity of the element on the Mulliken scale is:a)3.16b)0.0c)2.8d)4.16Correct answer is option 'C'. Can you explain this answer? for Chemistry 2024 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about If the ionization and electron gain enthalpy of an element are 275and86Kcalmol−1 respectively, then the electronegativity of the element on the Mulliken scale is:a)3.16b)0.0c)2.8d)4.16Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for Chemistry 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for If the ionization and electron gain enthalpy of an element are 275and86Kcalmol−1 respectively, then the electronegativity of the element on the Mulliken scale is:a)3.16b)0.0c)2.8d)4.16Correct answer is option 'C'. Can you explain this answer?.
Solutions for If the ionization and electron gain enthalpy of an element are 275and86Kcalmol−1 respectively, then the electronegativity of the element on the Mulliken scale is:a)3.16b)0.0c)2.8d)4.16Correct answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of If the ionization and electron gain enthalpy of an element are 275and86Kcalmol−1 respectively, then the electronegativity of the element on the Mulliken scale is:a)3.16b)0.0c)2.8d)4.16Correct answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
If the ionization and electron gain enthalpy of an element are 275and86Kcalmol−1 respectively, then the electronegativity of the element on the Mulliken scale is:a)3.16b)0.0c)2.8d)4.16Correct answer is option 'C'. Can you explain this answer?, a detailed solution for If the ionization and electron gain enthalpy of an element are 275and86Kcalmol−1 respectively, then the electronegativity of the element on the Mulliken scale is:a)3.16b)0.0c)2.8d)4.16Correct answer is option 'C'. Can you explain this answer? has been provided alongside types of If the ionization and electron gain enthalpy of an element are 275and86Kcalmol−1 respectively, then the electronegativity of the element on the Mulliken scale is:a)3.16b)0.0c)2.8d)4.16Correct answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice If the ionization and electron gain enthalpy of an element are 275and86Kcalmol−1 respectively, then the electronegativity of the element on the Mulliken scale is:a)3.16b)0.0c)2.8d)4.16Correct answer is option 'C'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















