Class 11 Exam > Class 11 Questions > what is hyperconjugation
Start Learning for Free
what is hyperconjugation
Most Upvoted Answer
what is hyperconjugation
In the formalism that separates bonds into σ and π types, hyperconjugation is the interaction of σ-bonds (e.g. C-H, C-C, etc.) with a π network.
This interaction is customarily illustrated by contributing structures, e.g. for toluene (below), sometimes said to be an example of "heterovalent" or "sacrificial hyperconjugation", so named because the contributing structure contains one two-electron bond less than the normal Lewis formula for toluene.
This interaction is customarily illustrated by contributing structures, e.g. for toluene (below), sometimes said to be an example of "heterovalent" or "sacrificial hyperconjugation", so named because the contributing structure contains one two-electron bond less than the normal Lewis formula for toluene.
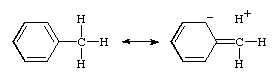
At present, there is no evidence for sacrificial hyperconjugation in neutral hydrocarbons.
The concept of hyperconjugation is also applied to carbenium ions and radicals, where the interaction is now between σ-bonds and an unfilled or partially filled π or p-orbital. A contributing structure illustrating this for the tert-butyl cation is:
The concept of hyperconjugation is also applied to carbenium ions and radicals, where the interaction is now between σ-bonds and an unfilled or partially filled π or p-orbital. A contributing structure illustrating this for the tert-butyl cation is:
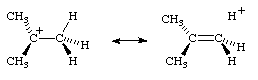 ce".
ce".This latter example is sometimes called an example of "isovalent hyperconjugation" (the contributing structure containing the same number of two-electron bonds as the normal Lewis formula).
Both structures are also examples of "double bond- no-bond resonance".
The interaction between filled π or p orbitals and adjacent antibonding σ* orbitals is referred to as "negative hyperconjugation", as for example in the fluoroethyl anion:
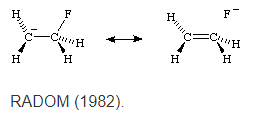
Community Answer
what is hyperconjugation
Hyperconjugation
Hyperconjugation is a concept in organic chemistry that explains the stability and reactivity of certain molecules or ions. It involves the delocalization of electrons through sigma bonds, which can influence the properties and behavior of organic compounds. Here, we will discuss hyperconjugation in detail, including its definition, mechanism, and applications.
Definition
Hyperconjugation refers to the interaction of electrons in a sigma bond with an adjacent empty or partially-filled orbital. This interaction occurs when a sigma bond is in proximity to a pi bond or an empty p orbital. The delocalization of electrons through sigma bonds leads to stabilization of the molecule or ion.
Mechanism
The concept of hyperconjugation is based on the molecular orbital theory. When a sigma bond is adjacent to a pi bond or an empty p orbital, the electrons in the sigma bond can interact with the empty or partially-filled orbital, resulting in overlap and delocalization of electron density.
This delocalization occurs through the overlap of the sigma bond orbital with the antibonding orbital of the adjacent pi bond or p orbital. It leads to the stabilization of the molecule or ion by spreading out the electron density and reducing the electron-electron repulsion.
Key Points:
- Hyperconjugation involves the interaction of electrons in sigma bonds with adjacent empty or partially-filled orbitals.
- It occurs through the overlap of the sigma bond orbital with the antibonding orbital of the adjacent pi bond or p orbital.
- The delocalization of electron density leads to stabilization of the molecule or ion.
Applications
Hyperconjugation has several important applications in organic chemistry. It helps explain the stability of certain molecules and ions, as well as their reactivity in various chemical reactions. Some key applications include:
1. Stability of carbocations: Hyperconjugation stabilizes carbocations by delocalizing the positive charge through adjacent sigma bonds. This stabilization reduces the reactivity of carbocations and influences their reactions.
2. Stereochemistry: Hyperconjugation affects the stability of different conformations of organic compounds. It can influence the preferred conformation of molecules by providing additional stabilization through delocalization of electrons.
3. Bond strength and acidity: Hyperconjugation can influence the strength of sigma bonds and the acidity of compounds. The delocalization of electrons through sigma bonds can strengthen the bond and increase acidity.
4. Reaction rates: The presence of hyperconjugation can affect the rates of certain chemical reactions. It can stabilize transition states and intermediates, leading to changes in reaction rates.
Key Points:
- Hyperconjugation has applications in explaining the stability and reactivity of molecules and ions.
- It helps explain the stability of carbocations, influences stereochemistry, and affects bond strength and acidity.
- Hyperconjugation can also impact reaction rates by stabilizing transition states and intermediates.
In conclusion, hyperconjugation is an important concept in organic chemistry that involves the delocalization of electrons through sigma bonds to stabilize molecules or ions. It has applications in understanding the stability, reactivity, and stereochemistry of organic compounds, as well as the strength of bonds and acidity. By considering hyperconjugation, chemists can gain insights into the behavior and properties of various organic molecules and ions.
Hyperconjugation is a concept in organic chemistry that explains the stability and reactivity of certain molecules or ions. It involves the delocalization of electrons through sigma bonds, which can influence the properties and behavior of organic compounds. Here, we will discuss hyperconjugation in detail, including its definition, mechanism, and applications.
Definition
Hyperconjugation refers to the interaction of electrons in a sigma bond with an adjacent empty or partially-filled orbital. This interaction occurs when a sigma bond is in proximity to a pi bond or an empty p orbital. The delocalization of electrons through sigma bonds leads to stabilization of the molecule or ion.
Mechanism
The concept of hyperconjugation is based on the molecular orbital theory. When a sigma bond is adjacent to a pi bond or an empty p orbital, the electrons in the sigma bond can interact with the empty or partially-filled orbital, resulting in overlap and delocalization of electron density.
This delocalization occurs through the overlap of the sigma bond orbital with the antibonding orbital of the adjacent pi bond or p orbital. It leads to the stabilization of the molecule or ion by spreading out the electron density and reducing the electron-electron repulsion.
Key Points:
- Hyperconjugation involves the interaction of electrons in sigma bonds with adjacent empty or partially-filled orbitals.
- It occurs through the overlap of the sigma bond orbital with the antibonding orbital of the adjacent pi bond or p orbital.
- The delocalization of electron density leads to stabilization of the molecule or ion.
Applications
Hyperconjugation has several important applications in organic chemistry. It helps explain the stability of certain molecules and ions, as well as their reactivity in various chemical reactions. Some key applications include:
1. Stability of carbocations: Hyperconjugation stabilizes carbocations by delocalizing the positive charge through adjacent sigma bonds. This stabilization reduces the reactivity of carbocations and influences their reactions.
2. Stereochemistry: Hyperconjugation affects the stability of different conformations of organic compounds. It can influence the preferred conformation of molecules by providing additional stabilization through delocalization of electrons.
3. Bond strength and acidity: Hyperconjugation can influence the strength of sigma bonds and the acidity of compounds. The delocalization of electrons through sigma bonds can strengthen the bond and increase acidity.
4. Reaction rates: The presence of hyperconjugation can affect the rates of certain chemical reactions. It can stabilize transition states and intermediates, leading to changes in reaction rates.
Key Points:
- Hyperconjugation has applications in explaining the stability and reactivity of molecules and ions.
- It helps explain the stability of carbocations, influences stereochemistry, and affects bond strength and acidity.
- Hyperconjugation can also impact reaction rates by stabilizing transition states and intermediates.
In conclusion, hyperconjugation is an important concept in organic chemistry that involves the delocalization of electrons through sigma bonds to stabilize molecules or ions. It has applications in understanding the stability, reactivity, and stereochemistry of organic compounds, as well as the strength of bonds and acidity. By considering hyperconjugation, chemists can gain insights into the behavior and properties of various organic molecules and ions.

|
Explore Courses for Class 11 exam
|

|
Similar Class 11 Doubts
what is hyperconjugation
Question Description
what is hyperconjugation for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about what is hyperconjugation covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for what is hyperconjugation.
what is hyperconjugation for Class 11 2025 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about what is hyperconjugation covers all topics & solutions for Class 11 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for what is hyperconjugation.
Solutions for what is hyperconjugation in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of what is hyperconjugation defined & explained in the simplest way possible. Besides giving the explanation of
what is hyperconjugation, a detailed solution for what is hyperconjugation has been provided alongside types of what is hyperconjugation theory, EduRev gives you an
ample number of questions to practice what is hyperconjugation tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


























