JEE Exam > JEE Questions > Ejection of the photoelectron from metal in t...
Start Learning for Free
Ejection of the photoelectron from metal in the photoelectric effect experiment can be stopped by applying 0.5 V when the radiation of 250 nm is used. The work function of the metal is:
- a)4.5 eV
- b)4 eV
- c)5.5 eV
- d)5 eV
Correct answer is option 'A'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Ejection of the photoelectron from metal in the photoelectric effect e...
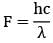
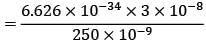
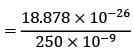
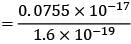
= 4.375 eV
Most Upvoted Answer
Ejection of the photoelectron from metal in the photoelectric effect e...
The photoelectric effect is the phenomenon in which electrons are ejected from the surface of a material when exposed to light of a certain frequency or wavelength. This effect can be explained using the concept of energy quantization. According to Einstein's theory, light can be thought of as particles called photons, and each photon carries a certain amount of energy.
The energy of a photon is given by the equation E = hf, where E is the energy, h is Planck's constant (6.626 x 10^-34 J s), and f is the frequency of the light. The energy required to remove an electron from the surface of a material is known as the work function (Φ) of the material. If the energy of a photon is greater than the work function, the electron will be ejected from the material.
In this question, the radiation used has a wavelength of 250 nm. To find the energy of the photons, we can use the equation c = fλ, where c is the speed of light (3 x 10^8 m/s), f is the frequency, and λ is the wavelength. Rearranging the equation, we get f = c/λ. Substituting the values, we find that the frequency of the radiation is approximately 1.2 x 10^15 Hz.
Now, let's consider the stopping potential applied to stop the ejection of the photoelectron. The stopping potential is the minimum potential difference required to stop the ejected electrons. The stopping potential is related to the maximum kinetic energy (Kmax) of the ejected electrons by the equation Kmax = eV, where e is the charge of an electron (1.6 x 10^-19 C) and V is the stopping potential.
Since the electrons are ejected from the metal, the energy of the photons must be greater than the work function of the metal. Therefore, we can write the equation E = Φ + Kmax, where E is the energy of the photon. Rearranging the equation, we get Kmax = E - Φ.
Since the stopping potential is applied to stop the ejection of the photoelectron, the maximum kinetic energy of the ejected electron will be equal to the energy of the photon (E). Therefore, we can write the equation E = eV.
Now, let's substitute the values into the equations. The energy of the photon is given by E = hf, where h is Planck's constant and f is the frequency. The work function of the metal is given by Φ = hf0, where f0 is the threshold frequency (the minimum frequency required to eject an electron from the metal). We can rearrange this equation to find Φ = (h/f0).
Substituting the values, we get E = hf = (6.626 x 10^-34 J s) x (1.2 x 10^15 Hz) = 7.951 x 10^-19 J. Similarly, Φ = (6.626 x 10^-34 J s) / (f0).
Now, let's find the stopping potential. We know that E = eV, so V = E / e = (7.951 x 10^-19 J) / (1.6 x 10^-19 C) = 4.969 V.
Therefore, the stopping potential required to stop the ejection of the photoelectron is 4.969 V. Since
The energy of a photon is given by the equation E = hf, where E is the energy, h is Planck's constant (6.626 x 10^-34 J s), and f is the frequency of the light. The energy required to remove an electron from the surface of a material is known as the work function (Φ) of the material. If the energy of a photon is greater than the work function, the electron will be ejected from the material.
In this question, the radiation used has a wavelength of 250 nm. To find the energy of the photons, we can use the equation c = fλ, where c is the speed of light (3 x 10^8 m/s), f is the frequency, and λ is the wavelength. Rearranging the equation, we get f = c/λ. Substituting the values, we find that the frequency of the radiation is approximately 1.2 x 10^15 Hz.
Now, let's consider the stopping potential applied to stop the ejection of the photoelectron. The stopping potential is the minimum potential difference required to stop the ejected electrons. The stopping potential is related to the maximum kinetic energy (Kmax) of the ejected electrons by the equation Kmax = eV, where e is the charge of an electron (1.6 x 10^-19 C) and V is the stopping potential.
Since the electrons are ejected from the metal, the energy of the photons must be greater than the work function of the metal. Therefore, we can write the equation E = Φ + Kmax, where E is the energy of the photon. Rearranging the equation, we get Kmax = E - Φ.
Since the stopping potential is applied to stop the ejection of the photoelectron, the maximum kinetic energy of the ejected electron will be equal to the energy of the photon (E). Therefore, we can write the equation E = eV.
Now, let's substitute the values into the equations. The energy of the photon is given by E = hf, where h is Planck's constant and f is the frequency. The work function of the metal is given by Φ = hf0, where f0 is the threshold frequency (the minimum frequency required to eject an electron from the metal). We can rearrange this equation to find Φ = (h/f0).
Substituting the values, we get E = hf = (6.626 x 10^-34 J s) x (1.2 x 10^15 Hz) = 7.951 x 10^-19 J. Similarly, Φ = (6.626 x 10^-34 J s) / (f0).
Now, let's find the stopping potential. We know that E = eV, so V = E / e = (7.951 x 10^-19 J) / (1.6 x 10^-19 C) = 4.969 V.
Therefore, the stopping potential required to stop the ejection of the photoelectron is 4.969 V. Since
Attention JEE Students!
To make sure you are not studying endlessly, EduRev has designed JEE study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in JEE.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
Ejection of the photoelectron from metal in the photoelectric effect experiment can be stopped by applying 0.5 V when the radiation of 250 nm is used. The work function of the metal is:a)4.5 eVb)4 eVc)5.5 eVd)5 eVCorrect answer is option 'A'. Can you explain this answer?
Question Description
Ejection of the photoelectron from metal in the photoelectric effect experiment can be stopped by applying 0.5 V when the radiation of 250 nm is used. The work function of the metal is:a)4.5 eVb)4 eVc)5.5 eVd)5 eVCorrect answer is option 'A'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Ejection of the photoelectron from metal in the photoelectric effect experiment can be stopped by applying 0.5 V when the radiation of 250 nm is used. The work function of the metal is:a)4.5 eVb)4 eVc)5.5 eVd)5 eVCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Ejection of the photoelectron from metal in the photoelectric effect experiment can be stopped by applying 0.5 V when the radiation of 250 nm is used. The work function of the metal is:a)4.5 eVb)4 eVc)5.5 eVd)5 eVCorrect answer is option 'A'. Can you explain this answer?.
Ejection of the photoelectron from metal in the photoelectric effect experiment can be stopped by applying 0.5 V when the radiation of 250 nm is used. The work function of the metal is:a)4.5 eVb)4 eVc)5.5 eVd)5 eVCorrect answer is option 'A'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Ejection of the photoelectron from metal in the photoelectric effect experiment can be stopped by applying 0.5 V when the radiation of 250 nm is used. The work function of the metal is:a)4.5 eVb)4 eVc)5.5 eVd)5 eVCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Ejection of the photoelectron from metal in the photoelectric effect experiment can be stopped by applying 0.5 V when the radiation of 250 nm is used. The work function of the metal is:a)4.5 eVb)4 eVc)5.5 eVd)5 eVCorrect answer is option 'A'. Can you explain this answer?.
Solutions for Ejection of the photoelectron from metal in the photoelectric effect experiment can be stopped by applying 0.5 V when the radiation of 250 nm is used. The work function of the metal is:a)4.5 eVb)4 eVc)5.5 eVd)5 eVCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Ejection of the photoelectron from metal in the photoelectric effect experiment can be stopped by applying 0.5 V when the radiation of 250 nm is used. The work function of the metal is:a)4.5 eVb)4 eVc)5.5 eVd)5 eVCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Ejection of the photoelectron from metal in the photoelectric effect experiment can be stopped by applying 0.5 V when the radiation of 250 nm is used. The work function of the metal is:a)4.5 eVb)4 eVc)5.5 eVd)5 eVCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for Ejection of the photoelectron from metal in the photoelectric effect experiment can be stopped by applying 0.5 V when the radiation of 250 nm is used. The work function of the metal is:a)4.5 eVb)4 eVc)5.5 eVd)5 eVCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of Ejection of the photoelectron from metal in the photoelectric effect experiment can be stopped by applying 0.5 V when the radiation of 250 nm is used. The work function of the metal is:a)4.5 eVb)4 eVc)5.5 eVd)5 eVCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Ejection of the photoelectron from metal in the photoelectric effect experiment can be stopped by applying 0.5 V when the radiation of 250 nm is used. The work function of the metal is:a)4.5 eVb)4 eVc)5.5 eVd)5 eVCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























