Class 12 Exam > Class 12 Questions > Prussian blue is formed when [1989]a)Ferrous ...
Start Learning for Free
Prussian blue is formed when [1989]
- a)Ferrous sulphate reacts with FeCl3
- b)Ferric chloride reacts with K4 [Fe(CN)6]
- c)Fer rous ammon ium sulphate reacts with FeCl3
- d)Ammonium sulphate reacts with FeCl3
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Prussian blue is formed when [1989]a)Ferrous sulphate reacts with FeCl...
Potassium ferrocyanide solution is added to Fe3+ ions in solution to give deep blue solution or precipitate.
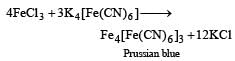
Most Upvoted Answer
Prussian blue is formed when [1989]a)Ferrous sulphate reacts with FeCl...
Prussian blue is a dark blue pigment that is commonly used in art and as a laboratory reagent. It is formed when ferric chloride (FeCl3) reacts with potassium ferrocyanide (K4[Fe(CN)6]).
Here is a detailed explanation of why option B is the correct answer:
1. Introduction:
Prussian blue is a coordination compound with the chemical formula Fe4[Fe(CN)6]3. It is an inorganic pigment that is known for its deep blue color. The compound is formed through a chemical reaction between ferric chloride and potassium ferrocyanide.
2. Ferric chloride (FeCl3):
Ferric chloride is a compound that contains iron in its +3 oxidation state. It is a yellowish-brown solid that is highly soluble in water. In aqueous solutions, it dissociates into Fe3+ ions and chloride ions (Cl-).
3. Potassium ferrocyanide (K4[Fe(CN)6]):
Potassium ferrocyanide is a coordination compound that contains iron in its +2 oxidation state. It is a yellow crystal that is also highly soluble in water. In aqueous solutions, it dissociates into K+ ions and the complex ion [Fe(CN)6]4-.
4. Reaction between FeCl3 and K4[Fe(CN)6]:
When ferric chloride reacts with potassium ferrocyanide, the following reaction occurs:
FeCl3 + 3K4[Fe(CN)6] → Fe4[Fe(CN)6]3 + 12KCl
In this reaction, the Fe3+ ions from ferric chloride combine with the [Fe(CN)6]4- ions from potassium ferrocyanide to form the Prussian blue complex ion Fe4[Fe(CN)6]3. The reaction also produces 12 moles of potassium chloride (KCl) as a byproduct.
5. Formation of Prussian blue:
The Prussian blue complex ion Fe4[Fe(CN)6]3 is responsible for the deep blue color of Prussian blue. It consists of a central iron atom surrounded by six cyanide ligands. The coordination geometry of the complex ion is octahedral.
6. Significance of the reaction:
The reaction between ferric chloride and potassium ferrocyanide is commonly used to synthesize Prussian blue in the laboratory. It is a simple and efficient method for producing the pigment.
In conclusion, Prussian blue is formed when ferric chloride reacts with potassium ferrocyanide. This reaction results in the formation of the Prussian blue complex ion Fe4[Fe(CN)6]3, which is responsible for the deep blue color of the pigment.
Here is a detailed explanation of why option B is the correct answer:
1. Introduction:
Prussian blue is a coordination compound with the chemical formula Fe4[Fe(CN)6]3. It is an inorganic pigment that is known for its deep blue color. The compound is formed through a chemical reaction between ferric chloride and potassium ferrocyanide.
2. Ferric chloride (FeCl3):
Ferric chloride is a compound that contains iron in its +3 oxidation state. It is a yellowish-brown solid that is highly soluble in water. In aqueous solutions, it dissociates into Fe3+ ions and chloride ions (Cl-).
3. Potassium ferrocyanide (K4[Fe(CN)6]):
Potassium ferrocyanide is a coordination compound that contains iron in its +2 oxidation state. It is a yellow crystal that is also highly soluble in water. In aqueous solutions, it dissociates into K+ ions and the complex ion [Fe(CN)6]4-.
4. Reaction between FeCl3 and K4[Fe(CN)6]:
When ferric chloride reacts with potassium ferrocyanide, the following reaction occurs:
FeCl3 + 3K4[Fe(CN)6] → Fe4[Fe(CN)6]3 + 12KCl
In this reaction, the Fe3+ ions from ferric chloride combine with the [Fe(CN)6]4- ions from potassium ferrocyanide to form the Prussian blue complex ion Fe4[Fe(CN)6]3. The reaction also produces 12 moles of potassium chloride (KCl) as a byproduct.
5. Formation of Prussian blue:
The Prussian blue complex ion Fe4[Fe(CN)6]3 is responsible for the deep blue color of Prussian blue. It consists of a central iron atom surrounded by six cyanide ligands. The coordination geometry of the complex ion is octahedral.
6. Significance of the reaction:
The reaction between ferric chloride and potassium ferrocyanide is commonly used to synthesize Prussian blue in the laboratory. It is a simple and efficient method for producing the pigment.
In conclusion, Prussian blue is formed when ferric chloride reacts with potassium ferrocyanide. This reaction results in the formation of the Prussian blue complex ion Fe4[Fe(CN)6]3, which is responsible for the deep blue color of the pigment.

|
Explore Courses for Class 12 exam
|

|
Prussian blue is formed when [1989]a)Ferrous sulphate reacts with FeCl3b)Ferric chloride reacts with K4 [Fe(CN)6]c)Fer rous ammon ium sulphate reacts with FeCl3d)Ammonium sulphate reacts with FeCl3Correct answer is option 'B'. Can you explain this answer?
Question Description
Prussian blue is formed when [1989]a)Ferrous sulphate reacts with FeCl3b)Ferric chloride reacts with K4 [Fe(CN)6]c)Fer rous ammon ium sulphate reacts with FeCl3d)Ammonium sulphate reacts with FeCl3Correct answer is option 'B'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Prussian blue is formed when [1989]a)Ferrous sulphate reacts with FeCl3b)Ferric chloride reacts with K4 [Fe(CN)6]c)Fer rous ammon ium sulphate reacts with FeCl3d)Ammonium sulphate reacts with FeCl3Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Prussian blue is formed when [1989]a)Ferrous sulphate reacts with FeCl3b)Ferric chloride reacts with K4 [Fe(CN)6]c)Fer rous ammon ium sulphate reacts with FeCl3d)Ammonium sulphate reacts with FeCl3Correct answer is option 'B'. Can you explain this answer?.
Prussian blue is formed when [1989]a)Ferrous sulphate reacts with FeCl3b)Ferric chloride reacts with K4 [Fe(CN)6]c)Fer rous ammon ium sulphate reacts with FeCl3d)Ammonium sulphate reacts with FeCl3Correct answer is option 'B'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Prussian blue is formed when [1989]a)Ferrous sulphate reacts with FeCl3b)Ferric chloride reacts with K4 [Fe(CN)6]c)Fer rous ammon ium sulphate reacts with FeCl3d)Ammonium sulphate reacts with FeCl3Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Prussian blue is formed when [1989]a)Ferrous sulphate reacts with FeCl3b)Ferric chloride reacts with K4 [Fe(CN)6]c)Fer rous ammon ium sulphate reacts with FeCl3d)Ammonium sulphate reacts with FeCl3Correct answer is option 'B'. Can you explain this answer?.
Solutions for Prussian blue is formed when [1989]a)Ferrous sulphate reacts with FeCl3b)Ferric chloride reacts with K4 [Fe(CN)6]c)Fer rous ammon ium sulphate reacts with FeCl3d)Ammonium sulphate reacts with FeCl3Correct answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Prussian blue is formed when [1989]a)Ferrous sulphate reacts with FeCl3b)Ferric chloride reacts with K4 [Fe(CN)6]c)Fer rous ammon ium sulphate reacts with FeCl3d)Ammonium sulphate reacts with FeCl3Correct answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Prussian blue is formed when [1989]a)Ferrous sulphate reacts with FeCl3b)Ferric chloride reacts with K4 [Fe(CN)6]c)Fer rous ammon ium sulphate reacts with FeCl3d)Ammonium sulphate reacts with FeCl3Correct answer is option 'B'. Can you explain this answer?, a detailed solution for Prussian blue is formed when [1989]a)Ferrous sulphate reacts with FeCl3b)Ferric chloride reacts with K4 [Fe(CN)6]c)Fer rous ammon ium sulphate reacts with FeCl3d)Ammonium sulphate reacts with FeCl3Correct answer is option 'B'. Can you explain this answer? has been provided alongside types of Prussian blue is formed when [1989]a)Ferrous sulphate reacts with FeCl3b)Ferric chloride reacts with K4 [Fe(CN)6]c)Fer rous ammon ium sulphate reacts with FeCl3d)Ammonium sulphate reacts with FeCl3Correct answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Prussian blue is formed when [1989]a)Ferrous sulphate reacts with FeCl3b)Ferric chloride reacts with K4 [Fe(CN)6]c)Fer rous ammon ium sulphate reacts with FeCl3d)Ammonium sulphate reacts with FeCl3Correct answer is option 'B'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















